Details of DPV and References
DPV NO: 310 December 1986
Family: Potyviridae
Genus: Potyvirus
Species: Iris fulva mosaic virus | Acronym: IFMV
Iris fulva mosaic virus
O. W. Barnett Clemson University, Clemson, South Carolina 29631, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Disease described by Travis (1957) and virus partially characterized by Barnett & Alper (1977).
A virus with filamentous particles about 770 nm long occurring naturally only in hybrids derived from Iris fulva. It has a restricted experimental host range and is transmissible by inoculation with sap and by aphids. Found in northeastern and western USA.
Main Diseases
Causes a mild mosaic, sometimes with leaf malformation, of Iris fulva hybrids (Apogon group: beardless, rhizomatous irises) (Travis, 1957; Barnett & Alper, 1977).
Geographical Distribution
Reported from northeastern USA (Barnett & Alper, 1977); also detected in western USA (O. W. Barnett, unpublished data).
Host Range and Symptomatology
The virus occurs naturally only in hybrids of Iris fulva. The only other iridaceous species infected experimentally were I. sibirica and Belamcanda chinensis (Barnett & Alper, 1977). In addition to these monocotyledonous species, Amaranthus caudatus (Amaranthaceae) and Chenopodium quinoa (Chenopodiaceae) can also be infected by inoculation with sap.
-
Diagnostic species
- Amaranthus caudatus, Chenopodium quinoa.
Chlorotic local lesions develop occasionally. - Belamcanda chinensis. Few chlorotic local lesions occur but the plants show a chronic
systemic mosaic which is usually distinct but may be very mild. Some of the younger leaves may curl
downward or twist as a result of necrotic streaks (Fig. 1).
The capsule is discoloured and malformed.
- Iris fulva hybrids. A faint mosaic or yellow streaking occurs on systemically infected leaves which also may twist. Plants are difficult to infect by inoculation with sap.
- Iris sibirica. Systemically infected leaves of inoculated seedlings develop long yellow streaks which soon become necrotic (Fig. 2). Infected plants usually die soon after necrosis develops.
- Iris fulva hybrids. A faint mosaic or yellow streaking occurs on systemically infected leaves which also may twist. Plants are difficult to infect by inoculation with sap.
-
Propagation and assay species
- Belamcanda chinensis,
which is readily infected by inoculation with sap, is a good source of inoculum and of virus for purification. It is also useful for whole plant infectivity assays.
Strains
None reported.
Transmission by Vectors
The virus from I. fulva described by Travis (1957) was transmitted in a non-persistent manner by the aphids Myzus persicae, Macrosiphum euphorbiae (= M. solanifolii) and Aphis fabae. The isolate characterized by Barnett & Alper (1977) was not transmitted by M. persicae, M. euphorbiae or A. craccivora but another isolate, closely related serologically, was transmitted by M. persicae.
Transmission through Seed
Not seed-borne in Belamcanda chinensis (Barnett & Alper, 1977).
Serology
A rabbit injected repeatedly over a 10-month period yielded antiserum which had a low titre in microprecipitin tests and contained antibodies to healthy plant material. Virus particles became coated with homologous antibody in immuno-electron microscopy tests (Ball & Brakke, 1968, modified by Langenberg, 1974) and reacted with homologous antiserum in Ouchterlony gel diffusion tests in the presence of 0.5% sodium dodecyl sulphate (Gooding & Bing, 1970). Reactions with the serum in DAS-ELISA were specific after absorption of the conjugate with healthy plant extracts (Lister, 1978).
Nucleic Acid Hybridization
Complementary DNA made to randomly primed nucleic acid (presumed to be RNA) from iris fulva mosaic virus did not hybridize with nucleic acid from iris severe mosaic virus (bearded iris mosaic virus) or from maize dwarf mosaic virus serotypes A or B in direct molecular hybridization tests done at 60°C in low salt (0.18 M NaCl) buffer (O. W. Barnett, unpublished data).
Relationships
The properties of iris fulva mosaic virus place it in the potyvirus group. Barnett & Alper (1977) found that its particles did not become coated with antibody to iris mild mosaic virus (Brunt, 1973; Barnett & Brunt, 1975), bearded iris mosaic virus (Barnett et al., 1971; Barnett & Brunt, 1975) or iris severe mosaic virus (see Brunt, 1973) in immuno-electron microscopy tests. Iris fulva mosaic virus reacted weakly with an antiserum to maize dwarf mosaic virus-B in DAS-ELISA (O. W. Barnett, unpublished data) but not with antiserum to the following other potyviruses: bean yellow mosaic, clover yellow vein, maize dwarf mosaic-A, soybean mosaic, papaya ringspot (type W) or watermelon mosaic-2.
Stability in Sap
In B. chinensis sap the virus was infective for 2 days but not 4 days at 27°C, for 10 min at 50° but not 60°C, and after diluting to 10-4 but not 10-5.
Purification
Barnett & Alper (1977) found that the following method, adapted from the procedures of Huttinga (1973), gave consistent yields of non-aggregated particles of iris fulva mosaic virus. Homogenize each 1 g infected leaves in 3 ml 0.1 M Tris (adjusted to pH 8.9 with thioglycollic acid) and 1.6 ml of a 1:1 mixture of carbon tetrachloride and chloroform, subject the aqueous phase to one or two cycles of differential centrifugation (1.5 h at 44,000 g; 10 min at 5000 g), and further purify the virus by rate zonal sucrose density gradient centrifugation.
Purification method 2 of Reddick & Barnett (1983), which involves the use of polyethylene glycol, Triton X-100, and equilibrium centrifugation in caesium sulphate, also consistently gave high yields of virus.
Properties of Particles
Purified preparations give a single light-scattering band in density gradient centrifugation. On analytical centrifugation the virus sedimented as a single symmetrical peak which broadened late in the run.
Sedimentation coefficient (s°20, w): c. 141 S (of questionable validity because of possible particle breakage).
A260/A280: 1.19; A260(max)/A247(min): 1.17 (both values corrected for light-scattering).
Particle Structure
Particles are slightly flexuous filaments c. 770 nm long (Fig. 3). The virus particles are flexuous in the presence of MgCl2 and straight in the presence of EDTA, effects opposite to those reported for other viruses of the potyvirus group (Govier & Woods, 1971). The particles appear swollen when stained with 2% sodium phosphotungstate, pH 7, but not when stained with 2% ammonium molybdate, pH 7, or 1% uranyl acetate, pH 3.
Particle Composition
Nucleic acid: 6% of particle weight (estimated from the A260/A280 ratio). Single nucleic acid component of about 2.9 x 106 daltons (O. W. Barnett, unpublished data).
Protein: Single polypeptide of about 32,000 daltons (O. W. Barnett, unpublished data).
Relations with Cells and Tissues
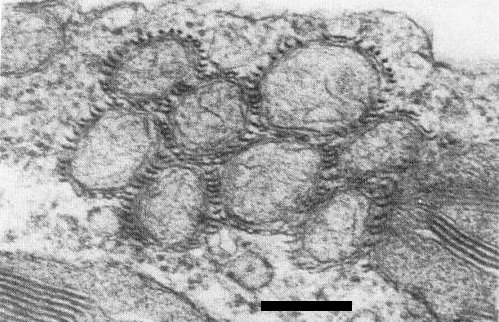
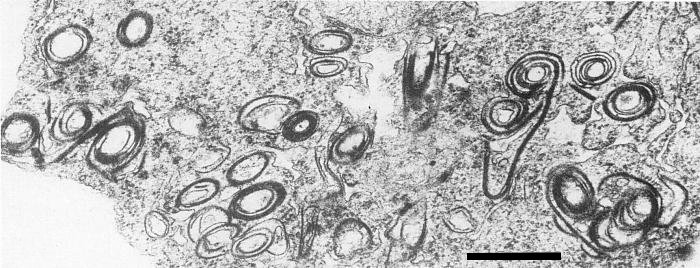
In sections of infected Belamcanda chinensis, virus particles are abundant in the cytoplasm of mesophyll cells. Occasionally mitochondria are aggregated (Fig. 4) with virus particles surrounding them. Cytoplasmic inclusions are present, consisting of both pinwheels and scrolls with laminated aggregates (Fig. 5); this places the virus in Edwardson's (1974) subdivision III of the potyvirus group.
Notes
Besides iris fulva mosaic virus, four other, serologically unrelated, potyviruses are found
in iris: iris severe mosaic,
iris mild mosaic and bean yellow mosaic viruses
(Brunt & Phillips, 1980;
Derks et al., 1985), and
turnip mosaic virus
(Inouye & Mitsuhata, 1978).
Iris severe mosaic virus was recently shown to be synonymous with bearded iris mosaic
virus
(Derks & Hollinger, 1986). Iris mild mosaic virus differs from iris fulva mosaic virus
in not infecting Belamcanda chinensis; although it infects Chenopodium quinoa locally,
it does so with ease throughout the year, whereas iris fulva mosaic virus infects it with difficulty
and only in some seasons. Bean yellow mosaic virus infects a number of standard test plant species,
e.g. Pisum sativum; turnip mosaic virus infects several species of Cruciferae and Lupinus
albus. Iris fulva mosaic virus is more easily confused with bearded iris mosaic isolates of iris
severe mosaic virus which, like it, occur in beardless irises and infect B. chinensis, although
inducing more severe symptoms. Thus strains of iris severe mosaic virus from Iris spuria induce
severe systemic leaf chlorosis from which B. chinensis plants do not recover and usually die;
other strains cause local symptoms and severe systemic mosaic followed by partial recovery or
symptomless systemic infection. Bearded iris mosaic virus is placed in
Edwardson's (1974) subdivision
II of the potyvirus group on the basis of the morphology of its cytoplasmic inclusions, whereas
iris fulva mosaic virus is placed in subdivision III. A potyvirus from a rhizomatous iris in Italy
reacted with antisera to both iris severe mosaic virus (bearded iris mosaic virus) and iris fulva
mosaic virus
(Lisa, 1980) but the inclusion bodies caused by this virus were like subdivision II
cytoplasmic inclusions.
Iris fulva mosaic virus is distantly related to maize dwarf mosaic virus-B and induces similar types of cytoplasmic inclusion but it does not infect maize (Zea mays), nor does maize dwarf mosaic virus-B infect Belamcanda chinensis.
Figures

Mosaic symptoms in Belamcanda chinensis. Note that one leaf is twisted as a result of virus infection.
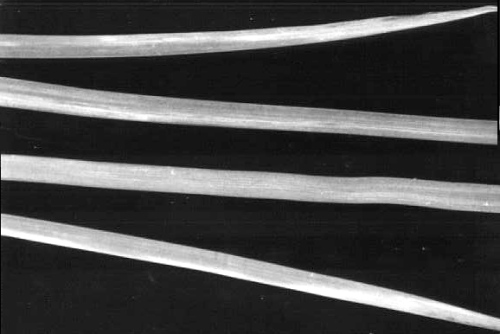
Leaves of Iris sibirica, infected by inoculation with sap, showing long, bright yellow-orange streaks (lighter areas).

Virus particles in 0.1 M Tris buffer with 0.05 M MgCl2 and stained with ammonium molybdate. Bar represents 150 nm.
References list for DPV: Iris fulva mosaic virus (310)
- Ball & Brakke, Virology 36: 152, 1968.
- Barnett & Alper, Phytopathology 67: 448, 1977.
- Barnett & Brunt, CMI/AAB Descriptions of Plant Viruses 147, 4 pp., 1975.
- Barnett, de Zoeten & Gaard, Phytopathology 61: 926, 1971.
- Brunt, CMI/AAB Descriptions of Plant Viruses 116, 4 pp., 1973.
- Brunt, Ann. appl. Biol. 87: 355, 1978.
- Brunt & Phillips, Acta Hort. 109: 503, 1980.
- Derks & Hollinger, Acta Hort. 177: 555, 1986.
- Derks, Hollinger & Vink-van den Abeele, Acta Hort. 164: 309, 1985.
- Edwardson, Monogr. Ser. Fla agric. Exp. Stn No. 4, 398 pp., 1974.
- Gooding & Bing, Phytopathology 60: 1293, 1970.
- Govier & Woods, J. gen. Virol. 13: 127, 1971.
- Huttinga, Neth. J. Pl. Path. 79: 125, 1973.
- Inouye & Mitsuhata, Noguku Kenkyu 57: 1, 1978.
- Langenberg, Phytopathology 64: 128, 1974.
- Lisa, Acta Hort. 110: 39, 1980.
- Lister, Phytopathology 68: 1383, 1978.
- Reddick & Barnett, Phytopathology 73: 1506, 1983.
- Travis, Phytopathology 47: 454, 1957.