Details of DPV and References
DPV NO: 330 September 1988
Family: Virgaviridae
Genus: Tobamovirus
Species: Pepper mild mottle virus | Acronym: PMMoV
Pepper mild mottle virus
C. Wetter Department of Botany, University of the Saarland, D-66 Saarbrücken, Germany
M. Conti Istituto di Fitovirologia Applicata, Via O. Vigliani, 104, 10135 Torino, Italy
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
McKinney (1952).
Synonyms
- Latent strain of tobacco mosaic virus
(McKinney, 1952,
1968)
- Samsun latent strain of tobacco mosaic virus (Greenleaf et al., 1964)
-
A virus with rod-shaped particles c. 312 x 18 nm, easily transmitted by mechanical inoculation and by handling during cultivation; also transmitted through contaminated seeds. No biological vector known. Of economic importance for pepper crops, particularly under glasshouses and plastic tunnels.
Main Diseases
Mild leaf chlorosis and growth reduction occur in naturally infected commercial pepper cultivars (Capsicum spp.). Fruits are small, malformed, mottled, and sometimes develop necrotic depressed areas (Fig. 1). Stunting is very severe in Capsicum spp. infected at early stages of growth (Fig. 3). In field crops, infection may reach 100% and the yield of marketable fruits is drastically reduced (Conti & Marte, 1983; Marte & Wetter, 1986).
Geographical Distribution
Occurs in North America, Australia, Japan and Europe, especially in Denmark, Iceland, England, France, Greece, Italy, The Netherlands and Spain (McKinney, 1952; Gebre Selassie et al., 1981; Nagai et al., 1981; Tóbiás et al., 1982; Paludan, 1982; Betti et al., 1982; Conti & Marte, 1983; Wetter et al., 1984; Pares, 1985; Brunt, 1986; Marte & Wetter, 1986).
Host Range and Symptomatology
The virus systemically infects all Capsicum spp. so far tested, including sweet pepper cultivars (e.g. ‘Lamuyo’ and ‘Yolo Wonder’) and hot peppers (C. baccatum, C. cardenasei, C. chacoense, C. chinense, C. eximium, C. frutescens, C. microcarpum, C. praetermissum, C. pubescens) which are immune or hypersensitive to tobacco mosaic and tomato mosaic viruses. Slight tolerance to the virus has been found only in a few Hungarian lines of C. pendulum (Conti, 1984). Many other species in the family Solanaceae are susceptible, but not tomato or Nicotiana glauca (McKinney, 1952; Greenleaf et al., 1964; Betti et al., 1982; Tóbiás et al., 1982; Wetter et al., 1984).
-
Diagnostic species
- Datura stramonium.
Small necrotic local lesions; no systemic infection. - Chenopodium amaranticolor, C. quinoa. Chlorotic local lesions; no systemic
infection.
- Capsicum chacoense, C. praetermissum. Severe top necrosis.
- Nicotiana glutinosa, N. sylvestris, N. tabacum cvs. White Burley and Xanthi-nc. Small necrotic local lesions; no systemic infection (Fig. 2).
- Capsicum chacoense, C. praetermissum. Severe top necrosis.
-
Propagation species
- The virus multiplies in the inoculated leaves of N. tabacum cv. Samsun but does not become systemic. N. clevelandii, N. debneyi and several Capsicum species may be used for propagation. C. frutescens is the best host for maintaining pure virus cultures.
-
Assay species
- D. stramonium, N. tabacum
cv. White Burley, and N. sylvestris. These species, however, do not reliably produce local lesions when sap of infected Capsicum spp. is used as inoculum, because of its high content of virus inhibitor.
Strains
Minor variants differ in their ability to induce a hypersensitive reaction on several differential cultivars of Capsicum spp. No serological differences could be detected between these isolates (Tóbiás et al., 1982; Marte & Wetter, 1986). An attenuated isolate inducing almost no symptoms in most pepper cultivars has been selected from ordinary pepper mild mottle virus by growing infected plants serially at 35°C. Young pepper seedlings inoculated with the attenuated isolate were protected against infection with severe field strains of pepper mild mottle virus but not against infection with tomato mosaic or type tobacco mosaic viruses (Nagai & Fukami, 1986).
Transmission by Vectors
No reports.
Transmission through Seed
Transmitted through 22% of seed of C. frutescens (McKinney, 1952) and through 29% of seed of C. annuum (Tosic et al., 1980). Virus transmissibility decreases with time of storage after seed harvest. The virus is present on the outer seed coat and rarely in the endosperm. Seedlings may be heavily infected during transplanting (up to 41%). The virus can be eliminated from the seed coats by soaking seeds in 4.2% calcium hypochlorite for 15 min, or in 10% trisodium phosphate for 30 min (Demski, 1981).
Serology
The virus is a good immunogen. Intravenous and/or intramuscular injections of a total of 10 mg virus with Freund’s incomplete adjuvant gave antiserum titres up to 1/4096 in slide precipitin tests. Indirect ELISA can be used for determining antiserum titres (up to 10-7) and for determining relationships to other tobamoviruses (Wetter et al., 1987).
Relationships
The properties of the virus place it in the tobamovirus group. Serologically, it is more closely related to bell pepper mottle, odontoglossum ringspot and tobacco mild green mosaic (U2) viruses than to tobacco mosaic and tomato mosaic viruses. Immunodiffusion tests and intragel absorption tests showed that pepper mild mottle virus antiserum contained a high concentration of pepper mild mottle virus-specific antibody (Fig. 7, Fig. 8) (Wetter et al., 1984, 1987). In the double antibody sandwich form of ELISA, a weak cross-reaction was found with some strains of tobacco mild green mosaic virus (e.g. U2) and with odontoglossum ringspot virus; no reaction was observed with tobacco mosaic or tomato mosaic viruses. Serological differentiation indices correlate with the degree of difference in the amino acid composition of the coat proteins. The virus is also remotely related to cucumber green mottle mosaic and sunn-hemp mosaic viruses when tested by indirect ELISA (Wetter et al., 1984, 1987).
Stability in Sap
In sap from N. clevelandii assayed on N. glutinosa or D. stramonium the virus lost infectivity after 10 min at 95°C or after dilution beyond 10-8 with distilled water (Wetter et al., 1984; McKinney, 1952).
Purification
Readily purified by many procedures such as salt or isoelectric precipitation and centrifugation. The following procedure is recommended: clarification of sap with a 1:1 mixture of chloroform and butan-1-ol, precipitation by adding polyethylene glycol (M. Wt 6000) to 4% (w/v) and NaCl to 4% (w/v) followed by three cycles of differential centrifugation. Yields may reach 1.3 g/kg tissue. Substantially monodisperse preparations can form colloidal crystals in vitro (Kreibig & Wetter, 1980).
Properties of Particles
Rate zonal centrifugation of purified virus or of clarified sap in sucrose produced a single band containing uniform particles c. 312 nm long.
Isoelectric point is about pH 3.7.
Specific extinction (A260 nm(0.1%, 1 cm)) uncorrected for light-scattering, is 3.18.
A260/A280: 1.11.
Particle Structure
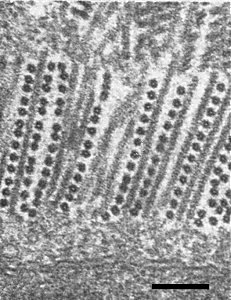
The particles are rigid rods, with a modal length of c. 312 nm and a width of c. 18 nm (Fig. 6). A central channel is visible and the protein subunits are arranged helically with a pitch of about 2.3 nm.
Particle Composition
Nucleic acid: No reports. Probably resembles that of the type strain of tobacco mosaic virus in size.
Protein: The coat protein consists of 158 amino acid residues and has the following gross composition (Wetter et al., 1984):
The absolute number of exchanges compared with tobacco mosaic virus is 32 (= 20%).
Asp
Thr
Ser
Glu
Pro
Gly
Ala
Cys
Val
Met
Ile
Leu
Tyr
Phe
His
Lys
Trp
Arg
18
20
10
18
6
8
17
1
14
1
5
16
4
7
0
2
2
9
Relations with Cells and Tissues
In pepper, the virus induces numerous angled-layer aggregate inclusions in the cytoplasm. The particles are orientated transversely to the long axis of these aggregates. Flat layers of virus particles are rotated against the next layer at an angle of about 60° (Fig. 4). In longitudinal sections, the inclusions appear as parallel lines interspersed with rows of cross-sectioned particles (Fig. 5). Hexagonal crystals typical of the type strain of tobacco mosaic virus also occur.
Notes
Pepper crops are frequently infected by other tobamoviruses such as tomato mosaic, tobacco mosaic and tobacco mild green mosaic (Wetter, 1984). These viruses can be distinguished from pepper mild mottle virus by the symptoms produced in diagnostic hosts and by serological tests. Differentiation from the more closely related bell pepper mottle virus is more difficult and is achieved reliably only by means of serological tests (Wetter et al., 1987). Mixed infections of peppers with two tobamoviruses occur frequently.
Figures

Leaf of tobacco cv. Xanthi-nc inoculated with sap of naturally infected Capsicum annuum cv. Pacific. Small necrotic lesions were induced by pepper mild mottle virus, few large lesions by tomato mosaic virus.

Transverse ultrathin section through angled-layer aggregates in the cytoplasm. Bar represents 300 nm.
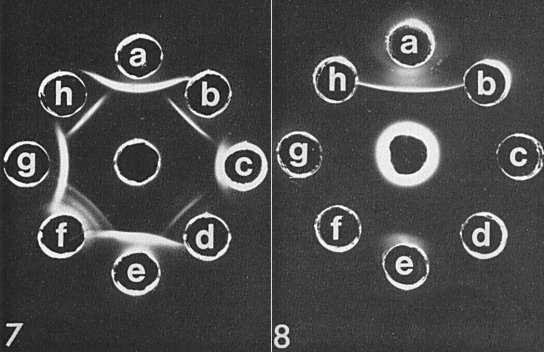
Immunodiffusion reaction (Fig. 7) of pepper mild mottle virus antiserum (central well) with the following viruses: a = pepper mild mottle; b = tomato mosaic; c = tobacco mild green mosaic; d = ribgrass mosaic; e = Ohio III strain of tobacco mosaic; f = odontoglossum ringspot; g = sunn-hemp mosaic; h = tobacco mosaic. Intragel absorption test (Fig. 8). Antiserum (central well) was absorbed with antigens b to h of Fig. 7. The reaction indicates the presence of a large proportion of antibodies specific to pepper mild mottle virus.

Immunodiffusion reaction (Fig. 7) of pepper mild mottle virus antiserum (central well) with the following viruses: a = pepper mild mottle; b = tomato mosaic; c = tobacco mild green mosaic; d = ribgrass mosaic; e = Ohio III strain of tobacco mosaic; f = odontoglossum ringspot; g = sunn-hemp mosaic; h = tobacco mosaic. Intragel absorption test (Fig. 8). Antiserum (central well) was absorbed with antigens b to h of Fig. 7. The reaction indicates the presence of a large proportion of antibodies specific to pepper mild mottle virus.
References list for DPV: Pepper mild mottle virus (330)
- Betti, Tanzi, Rubbini & Canova, Colture protette 12: 29, 1982.
- Brunt, in The Plant Viruses Vol. 2: p. 283, eds M. H. V. Van Regenmortel & H. Fraenkel-Conrat, 424 pp., New York: Plenum Press, 1986.
- Conti, Atti Miglioram. Genetico Peperone, Asti 177: 1984.
- Conti & Marte, Italia agric. 120: 132, 1983.
- Demski, Pl. Dis. 65: 723, 1981.
- Gebre Selassie, Dumas de Vaulx, Marchaux & Pochard, Agronomie 1: 853, 1981.
- Greenleaf, Cook & Heyn, Phytopathology 54: 1367, 1964.
- Kreibig & Wetter, Z. Naturf. 35c: 750, 1980.
- Marte & Wetter, Z. PflKrankh. PflPath. Pflschutz 93: 37, 1986.
- McKinney, Pl. Dis. Reptr 36: 184, 1952.
- McKinney, Pl. Dis. Reptr 52: 919, 1968.
- Nagai, Takeuchi & Tochihara, Ann. phytopath. Soc. Japan 47: 541, 1981.
- Nagai & Fukami, Bull. Chiba agric. Exp. Stn 27: 153, 1986.
- Paludan, Acta Hort. 127: 65, 1982.
- Pares, Ann. appl. Biol. 106: 469, 1985.
- Tóbiás, Rast & Maat, Neth. J. Pl. Path. 88: 257, 1982.
- Tosic, Sutic & Pesic, Phytopath. Z. 97: 10, 1980.
- Wetter, Pl. Dis. 68: 597, 1984.
- Wetter, Conti, Altschuh, Tabillion & Van Regenmortel, Phytopathology 74: 405, 1984.
- Wetter, Dore & Bernard, J. Phytopath. 119: 333, 1987.