Details of DPV and References
DPV NO: 337 September 1988
Family: Potyviridae
Genus: Potyvirus
Species: Bean common mosaic virus | Acronym: BCMV
This is a revised version of DPV 73
Bean common mosaic virus
F. J. Morales Centro Internacional de Agricultura Tropical, Apartado Aereo 6713, Cali, Colombia
L. Bos Research Institute for Plant Protection, P.O.B. 9060, 6700 GW Wageningen, The Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Stewart & Reddick (1917) and Pierce (1934).
Selected synonyms
- Bean mosaic virus (Stewart & Reddick, 1917)
- Bean virus 1 (Rev, appl. Mycol. 13: 488)
- Phaseolus virus 1 (Rev. appl. Mycol. 17: 52)
- Bean virus 1 (Rev, appl. Mycol. 13: 488)
-
A virus with flexuous filamentous particles c. 750 nm long and 12-15 nm wide, containing single-stranded RNA. It is transmitted by mechanical inoculation, by several aphid species in a non-persistent manner, and through seed and pollen. The virus induces the formation of cylindrical inclusions in the cytoplasm of infected cells. In nature it is mainly restricted to Phaseolus species, especially P. vulgaris, being found wherever this legume is grown.
Main Diseases
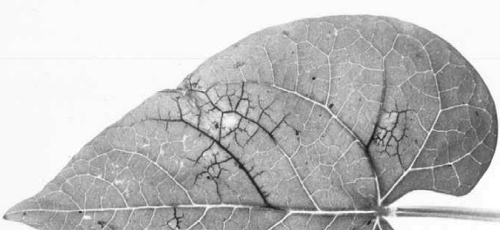
In Phaseolus vulgaris two main types of symptom occur, depending on virus strain and host genotype: 'common mosaic' (Fig. 1), often associated with leaf malformation, and 'black root' (Fig. 4), characterized by systemic necrosis and plant death (Drijfhout, 1978). The virus causes mosaic in most other susceptible Phaseolus species (Ainsworth, 1940; Quantz, 1961; Kaiser & Mossahebi, 1974; Anon., 1983).
Geographical Distribution
World-wide.
Host Range and Symptomatology
In nature, bean common mosaic virus is mainly found in Phaseolus species, predominantly P. vulgaris (Zaumeyer, 1957; Drijfhout, 1978) and occasionally in Lupinus luteus (Frencel & Pospieszny, 1979) and in wild legumes such as Rhynchosia minima (Meiners et al., 1978). Other reported but unconfirmed natural hosts are Vigna unguiculata (Zaumeyer, 1957), V. radiata (Kaiser & Mossahebi, 1974) and Crotalaria striata (Sarkar & Kulshreshtha, 1978). The experimental host range includes Cajanus cajan, Canavalia ensiformis, Cassia tora, Cicer arietinum, Crotalaria spectabilis, Cyamopsis tetragonoloba, Glycine max, Lens esculenta, Lupinus albus, L. angustifolius, Macroptilium lathyroides, M. atropurpureum, Melilotus alba, Phaseolus lathyroides, Sesbania exaltata, Trifolium incarnatum, T. subterraneum, Trigonella foenum-graecum, Vicia faba, V. sativa, V. villosa, Vigna angularis and Vigna unguiculata; some non-hosts are Arachis hypogaea, Lathyrus odoratus, Medicago sativa, Pisum sativum, Trifolium hybridum, T. pratense and T. repens (Pierce, 1934; Quantz, 1961; Gámez et al., 1970; Kaiser et al., 1971; Ordosgoitty, 1972; Kaiser & Mossahebi, 1974; Drijfhout & Bos, 1977). Five strains of the virus (US1, US2, US5, NL3 and NL4) did not induce visible symptoms in 14 cultivars of soybean (Glycine max) inoculated by mechanical means, and none of the five strains could be recovered from the inoculated soybean plants by back inoculation onto the bean cultivar Dubbele Witte (M. Castaño & F. J. Morales, unpublished data). Non-leguminous experimental hosts include Nicotiana clevelandii (Drijfhout & Bos, 1977) and N. benthamiana (Christie & Crawford, 1978).
-
Diagnostic species
- Phaseolus vulgaris.
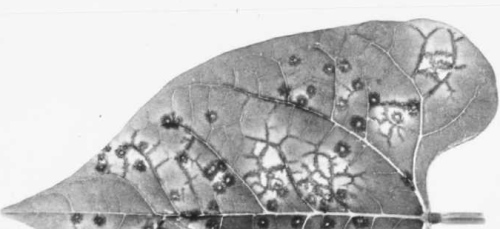
Some cultivars, such as Dubbele Witte and Stringless Green Refugee, are susceptible to all known strains of the virus: systemically infected plants usually exhibit leaf malformation and rolling (Fig. 2, Fig. 3) in addition to the characteristic vein-banding mosaic (Fig. 1) in which there are dark green areas along the main veins of upper leaves. Cultivars possessing the dominant necrosis gene never develop mosaic; some of them show pin-point necrotic local lesions (Fig. 6); in others this develops into local vein necrosis (Fig. 7); yet others show further systemic necrosis leading to the ‘black root’ syndrome (Fig. 4) which extends to the pods (Fig. 5) and results in plant death. A set of differential varieties possessing the dominant necrosis gene and/or different strain-specific recessive genes is available for strain identification (Drijfhout, 1978; Table 1).Propagation species
- Phaseolus vulgaris
(French bean). Cultivars that react with mosaic and are not severely affected by the virus are suitable propagation and maintenance hosts. For propagation, glasshouse-grown plants should be mechanically inoculated as soon as the primary leaves have expanded sufficiently (approximately 8-10 days after sowing) and should be kept for 10-14 days at moderate light and temperature before infected tissue is harvested. Inoculated leaves may contain as much virus as systemically infected leaves. The virus may be stored in infected seed of mosaic-reacting varieties but the virus particle concentration is lower than in leaf tissue.Assay species
- Phaseolus vulgaris.
Cvs Dubbele Witte and Stringless Green Refugee react with mosaic and/or leaf malformation to all strains of the virus. Cv. Monroe reacts with ring-shaped local lesions and non-vascular necrosis extending alongside the veins (Fig. 8) following manual inoculation of the primary leaves with any of at least five different strains of the virus (Saettler & Trujillo, 1972; Castaño et al., 1982). Cvs Cordon and Orfeo-INIA and experimental line IVT 7233 show pin-point local lesions without any systemic veinal necrosis (Fig. 6) when inoculated with necrosis-inducing strains (Drijfhout, 1978; F. J. Morales, unpublished data). Phaseolus lathyroides (Quantz, 1961) and Chenopodium quinoa are reported to show necrotic and chlorotic local lesions, respectively, but in C. quinoa the reaction depends upon the virus strain (Drijfhout & Bos, 1977).
Strains
Ten strains have been differentiated by their pathogenicity towards nine groups of P. vulgaris cultivars (Table 1).
Table 1. Classification of strains of bean common mosaic virus (Drijfhout, 1978)
| Bean common mosaic virus strains | |||||||||||
| Resistance Group | Differential Variety | US1 | NL7 | NL8 | US5 | NL6 | US2 | NL2 | NL3 | NL5 | NL4 |
| Mosaic-susceptible cultivars possessing recessive resistance genes | |||||||||||
| 1 | DubbeleWitte | +* | + | + | + | + | + | + | + | + | + |
| Stringless Green Refugee | + | + | + | + | + | + | + | + | + | + | |
| 2 | Redlands Greenleaf C | + | + | + | +w | + | +w | + | + | ||
| Puregold Wax | + | + | + | +w | + | +w | + | + | |||
| Imuna | +w | + | + | +w | + | +w | + | + | |||
| 3 | Redlands Greenleaf B | + | + | + | + | + | |||||
| Great Northern 123 | + | + | +w | +w | + | ||||||
| 4 | Sanilac | + | + | + | + | + | |||||
| Michelite 62 | + | + | + | + | + | ||||||
| Red Mexican 34 | + | + | + | + | + | ||||||
| 5 | Pinto 114 | + | + | + | + | ||||||
| 6 | Monroe | + | |||||||||
| Great Northern 31 | + | ||||||||||
| Red Mexican 35 | + | ||||||||||
| 7 | IVT 7214** | ||||||||||
| Necrosis-susceptible cultivars possessing the dominant necrosis gene | |||||||||||
| 8 | Widusa | + | +t | + | + | ||||||
| Black Turtle Soup | + | +t | + | + | |||||||
| 9a | Jubila | + | +t | + | + | ||||||
| 9b | Topcrop | +t | +t | + | + | ||||||
| Improved Tendergreen | +t | +t | + | + | |||||||
| 10 | Amanda | + | |||||||||
| 11 | IVT 7233 | ||||||||||
* Variety groups 1 to 7: Systemic chronic infection with obvious (+) or weak/inapparent (+w) mosaic symptoms.
Variety groups 8 to 10: Systemic necrosis (black root) at normal (20-26°C) temperatures (+) or increasing above 26°C (+t).
Blank spaces mean no systemic infection.
** IVT 7214 shows neither local nor systemic reactions to any of the known virus strains but is known through crossings not to contain the dominant necrosis gene.
Transmission by Vectors
The virus is transmitted in a non-persistent manner by several aphid species, notably Acyrthosiphon pisum, Aphis fabae and Myzus persicae (Kennedy et al., 1962; Zettler, 1966). Other reported vector species include Aphis gossypii, A. medicaginis, A. rumicis, Hyalopterus atriplicis, Macrosiphum ambrosiae, M. pisi and M. solanifolii (Zaumeyer, 1957). Most of these aphid species do not colonize P. vulgaris but transmit the virus efficiently as winged migrants. Aphids acquire the virus optimally in probes of 15-60 sec and transmit it within 1 min (Van der Want, 1954; Zettler, 1966, 1969)
Transmission through Seed
The high incidence of seed transmission is probably the most important factor affecting initial crop infection and the world-wide distribution of the virus. Depending upon the bean genotype and virus strain tested, up to 83% of the seed produced by infected plants may give rise to common mosaic-affected plants (Reddick & Stewart, 1919; Crowley, 1957; Schippers, 1963; Morales & Castaño, 1987). However, in some cultivars, such as Imuna, Great Northern 31 and Great Northern 123, seed transmission is inefficient with some virus strains. The bean cultivar Pinto 114 is highly resistant to seed infection by the US 2 strain (Morales & Castaño, 1987). Seed transmission of the virus is much decreased in plants infected after flowering (Nelson, 1932; Schippers, 1963). The virus is not appreciably affected during prolonged storage of the seed (up to 30 years: Pierce & Hungerford, 1929). The virus is located mostly in the embryo (Quantz, 1962; Provvidenti & Cobb, 1975); virus in the seed coat is inactivated during seed maturation (Hagita & Tamada, 1984). The virus may also enter seed via pollen (Reddick, 1931; Nelson & Down, 1933; Medina & Grogan, 1961). There is no seed transmission in genotypes possessing the dominant necrosis gene (Drijfhout, 1978). In tepary bean (P. acutifolius var. latifolius) the frequency of seed transmission ranged from 7 to 22% (Provvidenti & Cobb, 1975).
Serology
The virus is immunogenic in rabbits, and polyclonal antisera with titres over 1/2000 in precipitin tests can be obtained. These antisera can be used in ELISA and, after particle disruption with detergent, in agar diffusion tests (Bercks, 1959; Zaumeyer & Goth, 1964; Jafarpour et al., 1979; Morales, 1979). Monoclonal antibodies to bean common mosaic virus have also been used in ELISA (Wang, 1985). Depending on the test and degree of specificity of the antiserum, two main serological groups of strains can be distinguished according to their reactions with polyclonal antisera to five strains (Wang, 1983). Each monoclonal antibody tested was specific for two or more of the 22 isolates tested, but the necrosis-inducing strains NL3 and NL5 were differentiated as a distinct sero-group. One hybridoma cell line, however, produced a broad spectrum antibody which detected all 22 isolates tested, including NL3 and NL5 (Wang, 1985).
Relationships
The physical, chemical and biological properties of bean common mosaic virus place it in the potyvirus group. Edwardson (1974) classifies the virus in Subdivision I of the potyvirus group, according to the morphology of the cylindrical (pinwheel) inclusions induced in the cytoplasm of infected cells. Cross-protection has been reported to occur between some strains (Bercks, 1959; Silbernagel, 1969). Distant serological relationships are reported to 17 other potyviruses including the type member of the group, potato virus Y (Edwardson, 1974; Lima et al., 1979), and most of the potyviruses that infect legumes, such as soybean mosaic, blackeye cowpea mosaic and bean yellow mosaic viruses. Surprisingly, the potyvirus watermelon mosaic virus 2 is serologically related to most of the mosaic-inducing and necrosis-inducing strains of bean common mosaic virus (Edwardson, 1974; F. J. Morales, unpublished data), although it has an entirely different host range. Partial or complete cross-protection in plants has been found between bean common mosaic and bean yellow mosaic viruses (Grogan & Walker, 1948; Quantz, 1961) and between each of these viruses and soybean mosaic virus (Quantz, 1961).
Stability in Sap
Depending on the virus source, test conditions, and strain selected, the thermal inactivation point ranges from 50 to 60°C, the dilution end-point is between 10-3 and 10-4, and the virus retains its infectivity in sap for 1-4 days at room temperature (Bos, 1971).
Purification
Harvest systemically infected leaves 10 days after inoculation of the plants and homogenize each 100 g tissue in a mixture of 50 ml chloroform, 50 ml carbon tetrachloride and 200 ml cold 0.5 M potassium phosphate buffer, pH 7.5, containing 0.02 M sodium sulphite. Centrifuge the homogenized mixture for 5 min at 4000 g, discard the pellet and filter the supernatant fluid through glass wool. Add polyethylene glycol (PEG, M. Wt 6000) to 6% (w/v), stir for 1 h at 4°C, and recover the precipitated virus particles by centrifugation at 12,000 g for 10 min. Allow the pellet to resuspend undisturbed for at least 6 h, then clarify by centrifugation for 10 min at 12,000 g. Add a 20% PEG solution in 0.02 M Tris buffer, pH 8.2 (2 ml per 5 ml virus preparation), keep the mixture at 4°C for 1 h, then centrifuge at 17,000 g for 10 min. Resuspend the precipitate in 0.25 M potassium phosphate buffer, pH 7.5, and maintain overnight at 4°C before centrifuging at 12,000 g for 10 min. Add CsCl to 30% (w/w), centrifuge at 120,000 g for 17 h, recover the zone containing the virus particles and concentrate by centrifugation at 84,500 g for 60 min, or longer if they are fragmented (Morales, 1979).
Properties of Particles
Particle preparations contain a single sedimenting and buoyant density component.
Sedimentation coefficient, s20, w: 154-158 S for the US 1 and US 5 strains (Bravo, 1984; F.J. Morales, unpublished data).
A260/A280: 1.12-1.27 (uncorrected for light-scattering),
depending on the strain (Morales, 1979;
Bravo, 1984).
Buoyant density in CsCl: 1.31-1.32 g/cm3 (F. J. Morales, unpublished data).
Particle Structure
The particles are flexuous filaments (Fig. 9), 12-15 nm wide and 720-770 nm long, depending on the strain of the virus (Brandes & Quantz, 1955; Zaumeyer & Goth, 1964; Bravo, 1984).
Particle Composition
Nucleic acid: RNA, single-stranded, M. Wt about 35 x 106, and approximately 5% of the particle weight (Bravo, 1984).
Protein: 95% of particle weight, usually one main polypeptide species, of M. Wt (x 10-4) 3.2-3.5, estimated by electrophoresis in polyacrylamide/SDS gels. The US 1 strain often exhibits two protein bands, of M. Wt (x 10-4) 3.2 and 3.4 (Morales, 1979; Bravo, 1984). Virus preparations that have undergone limited proteolysis also contain a component of M. Wt 2.9 x 104.
Relations with Cells and Tissues
In infectivity tests and by autoradiography, bean common mosaic virus was found to infect a high proportion of the apical meristems (domes included) of P. vulgaris cv. Taylor, which bean yellow mosaic virus did not. This may explain why the former virus is seed-transmissible in P. vulgaris and the latter one not (Rubies-Autonell & Faccioli, 1985). Bean common mosaic virus induces characteristic cylindrical (pinwheel) inclusions in the cytoplasm of infected cells (Fig. 10). Scrolls and circles can also be observed in transverse sections. In longitudinal sections the central core appears as a bundle (Edwardson, 1974). These inclusions are made up of virus-coded non-particle protein of M. Wt about 68 x 103 and possess surface striations with a periodicity of about 5 nm. These inclusions can be discerned with the light microscope in stained epidermal strips (Zettler, 1969).
Notes
The virus usually gives distinctive symptoms in Phaseolus vulgaris but may be confused with several other viruses, whose presence may especially be suggested by unexpected reactions on the differential varieties listed in Table 1. Bean yellow mosaic virus has a much wider host range and readily produces symptoms in most cultivars of Pisum sativum and Vicia faba, and local and sometimes systemic symptoms in Chenopodium amaranticolor and C. quinoa. Blackeye cowpea mosaic virus also has a wider host range and readily infects several cowpea varieties. Soybean mosaic virus can be very pathogenic in some Phaseolus vulgaris cultivars (Costa et al., 1978), is seed-transmitted in soybean and P. vulgaris (Castaño & Morales, 1983) and, like bean common mosaic virus, has a narrow host range; however, it differs in infecting a much wider range of soybean cultivars. Also, eleven South American isolates of soybean mosaic virus induced necrosis in P. vulgaris cultivars not possessing the dominant necrosis gene for the necrotic reaction to bean common mosaic virus (Tamayo et al., 1980), and resistance to the two viruses in P. vulgaris is controlled by different genes (Provvidenti et al., 1982). The mosaic or mottling induced in P. vulgaris by the legume strains of cucumber mosaic virus, which are also seed-transmitted in P. vulgaris, usually differs from that induced by bean common mosaic virus, and the plants often recover; in addition cucumber mosaic virus induces numerous local lesions within 4-5 days in C. amaranticolor and C. quinoa (Bos & Maat, 1974; Jayasinghe, 1982). Cucumber mosaic virus, like southern bean mosaic virus, has spherical particles and a wide host range. Southern bean mosaic virus may be distinguished by its high thermal inactivation point (95°C) and dilution end-point (up to 4 x 10-6). Other viruses and pathogens can cause necrosis and plant death in P. vulgaris but the systemic vascular necrosis induced by necrotic strains of bean common mosaic virus can be recognized by the reticulate pattern exhibited mainly by young trifoliolate leaves. This net-like pattern is more evident when the young leaflets are held against the light, and can still be observed long after plant death.
Acknowledgements
(Photographs 1, 5, 7 and 8 courtesy Research Institute for Plant Protection, and Photograph 6 courtesy Institute for Horticultural Plant Breeding, Wageningen, The Netherlands; Photographs 2, 3, 4, 9 and 10 courtesy Centro Internacional de Agricultura Tropical, Cali, Colombia).
Figures
References list for DPV: Bean common mosaic virus (337)
- Ainsworth, Ann. appl. Biol. 27: 218, 1940.
- Anon., A. Rep. Centro Internacional de Agricultura Tropical, Bean Program, 1983: 46, 1983.
- Bercks, Phytopath. Z. 35: 105, 1959.
- Bos, CMI/AAB Descr. Pl. Viruses 73, 4 pp., 1971.
- Bos & Maat, Neth. J. Pl. Path. 80: 113, 1974.
- Brandes & Quantz, Naturwissenschaften 42: 588, 1955.
- Bravo, M.S. Thesis, Univ. Valle, 126 pp., 1984.
- Castaño & Morales, Fitopatol. Brasil. 8: 103, 1983.
- Castaño, Tamayo & Morales, Turrialba 32: 329, 1982.
- Christie & Crawford, Pl. Dis. Reptr 62: 20, 1978.
- Costa, da Costa, Almeida & Bulisani, Fitopatol. Brasil. 3: 27, 1978.
- Crowley, Aust. J. biol. Sci. 10: 449, 1957.
- Drijfhout, Genetic Interaction between Phaseolus vulgaris and Bean Common Mosaic Virus, Doctoral Thesis, Centre Agric. Publ. Docum., 98 pp., 1978. Also in Agric. Res.Rep., Wageningen 872, 1978.
- Drijfhout & Bos, Neth. J. Pl. Path. 83: 13, 1977.
- Edwardson, Monogr. Ser. Fla agric. Exp. Stn No. 4, 398 pp., 1974.
- Frencel & Pospieszny, Acta phytopath. Acad. Sci. hung. 14: 279, 1979.
- Gámez, Osores & Echandi, Turrialba 20: 397, 1970.
- Grogan & Walker, Phytopathology 38: 489, 1948.
- Hagita & Tamada, Bull. Hokkaido Exp. Stn 51: 83, 1984.
- Jayasinghe, Doctoral Thesis., Agric. Univ. Wageningen, 156 pp., 1982.
- Jafarpour, Shepherd & Grogan, Phytopathology 69: 1125, 1979.
- Kaiser & Mossahebi, Phytopathology 64: 1209, 1974.
- Kaiser, Mossahebi & Okhovat, Iran. J. Pl. Path. 7: 25, 1971.
- Kennedy, Day & Eastop, A Conspectus of Aphids as Vectors of Plant Viruses, London, Commonwealth Institute of Entomology, 114 pp., 1962.
- Lima, Purcifull & Hiebert, Phytopathology 69: 1252, 1979.
- Medina & Grogan, Phytopathology 51: 452, 1961.
- Meiners, Gillaspie, Lawson & Smith, Phytopathology 68: 283, 1978.
- Morales, Turrialba 29: 320, 1979.
- Morales & Castaño, Pl. Dis. 71: 51, 1987.
- Nelson, Tech. Bull. Mich. (St. Coll) agric. Exp. Stn No. 118, 71 pp., 1932.
- Nelson & Down, Phytopathology 23: 25, 1933.
- Ordosgoitty, Agron. trop. 22: 29, 1972.
- Pierce, Phytopathology 24: 87, 1934.
- Pierce & Hungerford, Phytopathology 19: 605, 1929.
- Provvidenti & Cobb, Pl. Dis. Reptr 59: 966, 1975.
- Provvidenti, Gonsalves & Ranalli, J. Hered. 73: 302, 1982.
- Quantz, Phytopath. Z. 43: 79, 1961.
- Quantz, NachrBl. dt. PflSchutzdienst., Stuttg. 14: 49, 1962.
- Reddick, Rapp. 2me Congr. int. Path. Comp. 1: 363, 1931.
- Reddick & Stewart, Phytopathology 9: 445, 1919.
- Rubies-Autonell & Faccioli, Phytopath. Mediterranea 24: 241, 1985.
- Saettler & Trujillo, Phytopathology 62: 489, 1972.
- Sarkar & Kulshreshtha, Curr. Sci. 47: 241, 1978.
- Schippers, Acta bot. néerl. 12: 433, 1963.
- Silbernagel, Phytopathology 59: 1809, 1969.
- Stewart & Reddick, Phytopathology 7: 61, 1917.
- Tamayo, Gomez & Morales, Fitopat. Colomb. 9: 71, 1980.
- Van der Want, Meded. Inst. Plantenziektenk. Onderz. No. 85, 84 pp., 1954.
- Wang, M.S. Thesis. Wash. St. Univ., 52 pp. 1983.
- Wang, Ph.D. Thesis, Wash. St. Univ., 189 pp. 1985.
- Zaumeyer, Tech. Bull. U.S. Dep. Agric. No. 868, 255 pp., 1957.
- Zaumeyer & Goth, Phytopathology 54: 1378, 1964.
- Zettler, Diss. Abstr. Sect. B. 27: 1696, 1966.
- Zettler, Phytopathology 59: 1109, 1969.