Details of DPV and References
DPV NO: 341 December 1989
Family: Potyviridae
Genus: Potyvirus
Species: Maize dwarf mosaic virus | Acronym: MDMV
Maize dwarf mosaic virus
R. E. Ford Department of Plant Pathology, University of Illinois, Urbana, IL 61801, USA
M. Tosic Faculty of Agriculture, University of Belgrade, Beograd-Zemun 11080, Yugoslavia
D. D. Shukla CSIRO, Division of Biotechnology, Parkville, Victoria 3052, Australia
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Janson & Ellett (1963) and Williams & Alexander (1965). Previously included in Description No. 88 as a strain of sugarcane mosaic virus (Pirone, 1972), but now shown to be an independent member of the potyvirus group (Shukla et al., 1989c).
Synonym
- Maize mosaic virus (Panjan, 1960)
-
A virus with flexuous filamentous particles c. 750 nm long and 13 nm in diameter containing single-stranded RNA. It infects numerous species in the Gramineae, and induces the formation of cytoplasmic, cylindrical (pinwheel and scroll) inclusions in host cells. It is transmissible mechanically, and by several aphid species in a non-persistent manner.
Main Diseases
Field and sweet corn (maize; Zea mays), grain and fodder sorghum (Sorghum bicolor), and Johnsongrass (S. halepense) are important natural hosts, developing mosaic (Fig. 1) and occasional necrotic foliage symptoms. Genetic resistance in maize is available in several inbred lines e.g. Pa 405 (Johnson et al., 1966) and Ill A, (MacKenzie et al., 1966).
Geographical Distribution
Possibly occurs wherever maize and sorghum are grown throughout the world except that it is not reported from Australia. However, in many instances it is difficult to determine from the literature whether the cause of a mosaic disease of maize in a particular country was maize dwarf mosaic virus or sugarcane mosaic virus.
Host Range and Symptomatology
The natural host range is restricted to maize, sorghum and Johnsongrass which develop mosaic and occasionally dwarfing symptoms. Sap transmission tests show that most species of Gramineae are susceptible, some being infected symptomlessly (Ford, 1967; Ford & Tosic, 1972; Tosic & Ford, 1972; Rosenkranz, 1983, 1987). Cultivated wheat, barley, oat, rye and rice are non-hosts.
-
Diagnostic species
- Zea mays
(maize). Seedlings of most cultivars are readily infected and show systemic mosaic symptoms (Fig. 2). - Sorghum bicolor (sorghum). Different sorghum cultivars are readily infected. Systemic mosaic (Fig. 3) and/or necrosis are produced (Tosic et al., 1990).
-
Propagation species:
- Johnsongrass (Sorghum halepense) is a perennial ideal for long-term
maintenance of strains.
- Sweet corn and sorghum cvs are good sources of virus for purification.
-
Assay species
- Sorghum cv. Atlas develops necrotic local lesions and bright systemic mosaic within 1 week (Tosic & Ford, 1972, 1983; Persley et al., 1985; Tosic et al., 1990). Assay for either local lesions or systemic symptoms is also done in other sorghum cvs such as SA 8735, BTX 3197 and Rio, and in sweet corn.
Strains
Five strains of maize dwarf mosaic virus, A (the type strain), C, D, E and F
(Louie & Knoke, 1975),
are still recognized. These strains are distinguished by
their characteristic symptoms in inbred maize line N 20, differential responses in
several other inbred maize lines, and frequencies of transmission by Acyrthosiphon
pisum and Myzus persicae. The strains are indistinguishable in particle
length, serological reactions with antiserum to maize dwarf mosaic virus strain A,
stability in sap and longevity of infectivity in detached leaves
(Louie & Knoke, 1975).
Maize dwarf mosaic virus strains B (MacKenzie et al., 1966) and O (McDaniel & Gordon, 1985) are now classified as strains of sugarcane mosaic virus and Johnsongrass mosaic virus, respectively (Shukla & Teakle, 1989; Shukla et al., 1989c; Teakle et al., 1989).
Transmission by Vectors
Maize dwarf mosaic virus is non-persistently transmitted after acquisition and inoculation feeding times of only a few minutes and can be retained up to 1-2 h by aphids from three subfamilies: Aphidinae, Lachninae and Drepanosiphinae (Nault & Knoke, 1981). Aphid species listed as vectors include, from most to least efficient: Schizaphis graminum, Aphis maidiradicis, Aphis craccivora, Aphis fabae, Acyrthosiphon pisum, Myzus persicae, Aphis gossypii, Therioaphis maculata, Sitobion (Macrosiphum) avenae, Rhopalosiphum padi, Rhopalosiphum poae, Macrosiphum euphorbiae, Rhopalosiphum maidis, Brevicoryne brassicae and Rhopalosiphum fitchii (Knoke & Louie, 1981). Many factors, such as the biotype of the aphid species, the concentration of the virus, the age of the virus source and the strain of the virus, influence the frequency of transmission (Tu & Ford, 1971; Thongmeearkom et al., 1976; Berger et al., 1983).
Transmission through Seed
Maize dwarf mosaic virus is seed-transmitted in dent corn at frequencies from 0.007% to 0.4% (Shepherd & Holdeman, 1965; Williams et al., 1968; Hill et al., 1974; Tosic & Sutic, 1977; Mikel et al., 1984).
Serology
Antisera with homologous titres of 1/128-1/256 have been produced by injecting rabbits with purified preparations of particles (Shepherd, 1965; Snazelle et al., 1971; Tosic & Ford, 1974; Jarjees & Uyemoto, 1984). These antisera give flocculent precipitates when mixed with purified virus preparations in microprecipitin tests, but granular precipitates when mixed with crude sap from infected plants (M. Tosic & R. E. Ford, unpublished data); it is not known whether this is due to the presence of broken particles or free protein in the sap. Antisera can also be used in enzyme-linked immunosorbent assay (Jarjees & Uyemoto, 1984), immunosorbent electron microscopy (Derrick, 1975; Giorda et al., 1986) and electro-blot immunoassay (Shukla et al., 1989b, 1989c). Virus-specific monoclonal antibodies have also been produced (Hill et al., 1984).
Relationships
Maize dwarf mosaic virus, with its filamentous particles c. 750 nm long, cylindrical (pinwheel) inclusions in cells (Fig. 5) and mechanical and aphid transmissibility, belongs to the potyvirus group. On the basis of an apparent distant serological relationship, strains of maize dwarf mosaic virus were thought to belong to sugarcane mosaic virus (Shepherd, 1965; Snazelle et al., 1971; Pirone, 1972; Jarjees & Uyemoto, 1984). However, recent tests by electro-blot immunoassay using polyclonal antibodies directed to the virus-specific N-termini of coat proteins have shown that U.S. maize dwarf mosaic virus strains A, D, E and F are unrelated to U.S. strains B and O, sugarcane mosaic virus strains A, B, D, E, H, I and M, and Australian sugarcane mosaic virus strains SC, BC and Sabi (Shukla et al., 1989c). The distant relationships observed and reported previously are now known to be due to antibodies directed to the conserved core regions (devoid of N and C termini) of the coat proteins, which show high sequence homology throughout the potyvirus group (Shukla et al., 1988b, 1989b).
Stability in Sap
The type strain has a thermal inactivation point of 55°C, a dilution end-point of 10-5 and longevity in vitro at 2-3°C of 192 h (Williams & Alexander, 1965). Other isolates of maize dwarf mosaic virus strain A originating in different regions of USA had thermal inactivation points between 56 and 58°C, longevity in vitro of up to 72 h and dilution end-points between 1 x 10-3 and 20 x 10-3 (Tosic & Ford, 1974),
Purification
1. (Shepherd, 1965).
Homogenize tissue from plants infected for 3-4 weeks in 0.05 M
sodium citrate containing 0.05% mercaptoethanol (1 g tissue/1 ml) and strain through
cheesecloth. Emulsify the homogenate with chloroform (1:1 v/v) and centrifuge at low
speed. Filter the aqueous phase through a pad of glass wool and ultracentrifuge for
90 min at 30,000 rev./min; resuspend pellets in about 1/50th the initial volume of
0.005 M borate, pH 8.2. Purify the virus further by sucrose density gradient
centrifugation.
2. Tosic et al. (1974)
compared several reported methods and found variable
results with different strains.
3. (Von Baumgarten & Ford, 1981). Homogenize tissue from plants infected for 10 to 12 days in 60 mM sodium phosphate buffer, pH 9.0, containing 10 mM NaDIECA (1 g tissue/4 ml) and express sap through cheesecloth. Add chloroform to 5% (v/v), emulsify and centrifuge at low speed. Add NaCl to 0.3 M and PEG M. Wt 6000 to 4% (w/v), adjust to pH 7.0, incubate for 30 min and centrifuge at low speed. Resuspend the pellet in 50 mM sodium phosphate, pH 8.0, containing 10 mM EDTA and centrifuge at low speed. Concentrate particles by ultracentrifugation through a 30% sucrose cushion. Purify further by centrifugation in a linear sucrose (10 to 40%) or CsCl gradient.
Properties of Particles
A sedimentation coefficient of 176 ± 5 S, buoyant density in CsCl of 1.3421 (Tosic & Ford, 1974) and a A280/A260 ratio of 0.82 (Langenberg, 1973) has been reported for strain A.
Particle Structure
Particles are flexuous filaments (Fig. 4) c. 750 nm long and 13 nm wide (Williams & Alexander, 1965).
Particle Composition
Nucleic acid: A single ssRNA species of c. 3.32 x 106 daltons, 50.7°C Tm and 23.3% hyperchromicity (Berger et al., 1988, 1989), representing c. 5% of the particle weight (Jones & Tolin, 1972).
Protein: A single polypeptide species of apparent M. Wt 30,700 daltons for a native preparation, or 35,500 daltons for a carboxymethylated preparation (Von Baumgarten & Ford, 1981; Jensen et al., 1986). Amino acid composition: ala 23, arg 14, asx 26, cys 1, glx 37, gly 18, his 7, ile 7, leu 14, lys 22, met 11, phe 8, pro 8, ser 15, thr 19, trp 4, tyr 9, val 13 (Von Baumgarten & Ford, 1981).
Genome Properties
The genomic constitution of maize dwarf mosaic virus is presumed to be similar to that of other potyviruses. The RNA of maize dwarf mosaic virus strain A is polyadenylated (Berger et al., 1989) probably (by analogy with other potyviruses) at the 3' end, and preceded by a short untranslated region. The coat protein cistron is immediately upstream of this untranslated region (J. Jilka & J. M. Clark, unpublished data). By analogy with the genomic RNA molecules of other potyviruses, that of maize dwarf mosaic virus strain A probably encodes, in addition to the coat protein, a cylindrical inclusion protein, a helper component protein, two nuclear inclusion proteins, and possibly a genome-linked protein.
Relations with Cells and Tissues
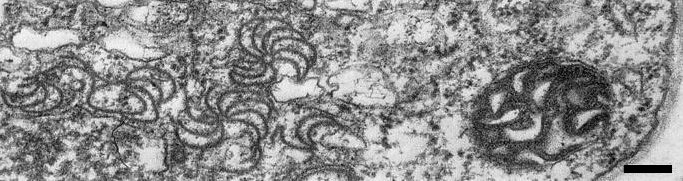
The virus becomes systemic in most hosts. Virus particles and cytoplasmic inclusions of ‘pinwheel’ and ‘scroll’ types (Fig. 5), characteristic of Edwardson’s (1974) subdivision I of the potyvirus group have been found in cells of Zea mays, Sorghum bicolor and S. halepense after infection with maize dwarf mosaic virus strains A, D and F (Krass & Ford, 1969; Moline, 1972; Langenberg & Schroeder, 1973; Edwardson, 1974). Membrane-bound micro-inclusion bodies occur between the plasmalemma and cell wall, and chloroplasts may be swollen (Krass & Ford, 1969).
Notes
Recent investigation of the taxonomy of aphid-borne potyviruses infecting species of Gramineae (Shukla et al., 1989c) has shown that virus isolates previously included as strains of sugarcane mosaic virus (Pirone, 1972) in fact comprise four distinct potyviruses. One of them is maize dwarf mosaic virus (U.S. strains A, D, E and F). The other three are Johnsongrass mosaic virus (formerly Australian sugarcane mosaic virus strain JG, U.S. maize dwarf mosaic virus strains O and Kansas 1; Jarjees & Uyemoto, 1984; Shukla & Teakle, 1989), sugarcane mosaic virus (U.S. maize dwarf mosaic virus strain B, sugarcane mosaic virus strains A, B, D and E, and Australian sugarcane mosaic virus strains SC, BC and Sabi; Teakle et al., 1989), and sorghum mosaic virus (U.S. sugarcane mosaic virus strains H, I and M; Shukla et al., 1989c). These four viruses induce similar symptoms in some hosts, have host ranges usually restricted to the Gramineae and have some aphid vectors in common. However, they are serologically unrelated or only distantly related (Shukla & Gough, 1984; Jarjees & Uyemoto, 1984; McDaniel & Gordon, 1985; Giorda et al., 1986; Shukla et al., 1989c). Previous reports of serological relationships among these viruses and their strains are caused by the presence in polyclonal antisera of antibodies to epitopes in the conserved core regions of potyvirus coat proteins; these antibodies recognize most potyviruses (Shukla et al., 1988b; 1989a, 1989b, 1989c). The viruses also differ considerably in the amino acid compositions and amino acid sequences of their coat proteins (Hill et al., 1973, Gough & Shukla, 1981; Von Baumgarten & Ford, 1981; Shukla et al., 1987; Jilka, 1990) and can be distinguished readily by high performance liquid chromatography of coat protein digests (Shukla et al., 1988a; N. M. McKern & D. D. Shukla, unpublished data). The four viruses can also be distinguished by their reactions on certain sorghum inbred lines (Teakle & Grylls, 1973; Persley et al., 1985; Giorda et al., 1986; Shukla & Teakle, 1989; Teakle et al., 1989; Tosic et al., 1990). Maize dwarf mosaic virus induces mosaic and necrotic spots in systemically infected leaves of sorghum cv. Rio whereas the other three viruses induce either mosaic or symptomless infection. Johnsongrass mosaic virus induces necrotic red stripes in sorghum lines OKY 8 and SA 8735 whereas the other three viruses induce only mosaic symptoms. Strains of sugarcane mosaic virus induce necrosis of new leaves of Atlas sorghum whereas sorghum mosaic virus induces typical red leaf symptoms, and the other two viruses induce only mosaic symptoms.
Figures

Mosaic symptoms in a plant of Zea mays (sweet corn) infected with maize dwarf mosaic virus strain A.

Mosaic symptoms in a leaf of Zea mays (Pfisters PAG SX60 hybrid) infected by maize dwarf mosaic virus strain A (right) compared with a healthy leaf (left).

Mosaic symptoms in leaves of Sorghum bicolor cv. Grazer: (left), healthy, (centre), infected by maize dwarf mosaic virus strain A; (right) infected with maize dwarf mosaic virus strain B, recently reclassified by Shukla et al. (1989c) as sugarcane mosaic virus.
References list for DPV: Maize dwarf mosaic virus (341)
- Berger, Toler & Harris, Pl. Dis. 67: 496, 1983.
- Berger, Luciano, Thornberry, Benner, Hill & Zeyen, Phytopathology 78: 1537, 1988.
- Berger, Luciano, Thornberry, Benner, Hill & Zeyen, J. gen. Virol. 70: 1845, 1989.
- Derrick, Proc. Am. phytopath. Soc. 2: 42, 1975.
- Edwardson, Monogr. Ser. Fla agric. Exp. Stn No. 4, 398 pp., 1974.
- Ford, Phytopathology 57: 450, 1967.
- Ford & Tosic, Phytopath. Z. 75: 315, 1972.
- Giorda, Toler & Miller, Pl. Dis. 70: 624, 1986.
- Gough & Shukla, Virology 111: 455, 1981.
- Hill, Ford & Benner, J. gen. Virol. 20: 327, 1973.
- Hill, Martinson & Russell, Crop Sci. 14: 232, 1974.
- Hill, Hill & Durand, J. gen. Virol. 65: 525, 1984.
- Janson & Ellett, Pl. Dis. Reptr 47: 1107, 1963.
- Jarjees & Uyemoto, Ann. appl. Biol. 104: 497, 1984.
- Jensen, Long-Davidson & Seip, Phytopathology 76: 528, 1986.
- Jilka, Ph.D. Thesis, University of Illinois, Urbana, USA, 160 pp., 1990.
- Johnson, McGahen, Craig & Sperling, Prog. Rep. Pennsylvania agric. Exp. Stn No. 268, 34 pp., 1966.
- Jones & Tolin, Phytopathology 62: 812, 1972.
- Knoke & Louie, South. Coop. Ser. Bull. No. 247: 92, 1981.
- Krass & Ford, Phytopathology 59: 431, 1969.
- Langenberg, Phytopathology 63: 149, 1973.
- Langenberg & Schroeder, Phytopathology 63: 1066, 1973.
- Louie & Knoke, Pl. Dis. Reptr 59: 518, 1975.
- McDaniel & Gordon, Pl. Dis. 69: 602, 1985.
- MacKenzie, Wernham & Ford, Pl. Dis. Reptr 50: 814, 1966.
- Mikel, D'Arcy & Ford, Phytopath. Z. 110: 185, 1984.
- Moline, Phytopathology 62: 1109, 1972.
- Nault & Knoke, South. Coop. Ser. Bull. No. 247: 77, 1981.
- Panjan, Zast. Bilja 62: 3, 1960.
- Persley, Henzell, Greber & Teakle, Pl. Dis. 69: 1046, 1985.
- Pirone, CMI/AAB Descr. Pl. Viruses 88, 4 pp, 1972.
- Rosenkranz, Phytopathology 73: 1314, 1983.
- Rosenkranz, Phytopathology 77: 598, 1987.
- Shepherd, Phytopathology 55: 1250, 1965.
- Shepherd & Holdeman, Pl. Dis. Reptr 49: 468, 1965.
- Shukla & Gough, Pl. Dis. 68: 204, 1984.
- Shukla & Teakle, AAB Descr. Pl. Viruses 340, 5 pp., 1989.
- Shukla, Gough & Ward, Arch. Virol. 96: 59, 1987.
- Shukla, McKern, Gough, Tracy & Letho, J. gen. Virol. 69: 493, 1988a.
- Shukla, Strike, Tracy, Gough & Ward, J. gen. Virol. 69: 1497, 1988b.
- Shukla, Ford, Tosic, Jilka & Ward, Arch. Virol. 105: 143, 1989a.
- Shukla, Jilka, Tosic & Ford, J. gen. Virol. 70: 13, 1989b.
- Shukla, Tosic, Jilka, Ford, Toler & Langham, Phytopathology 79: 223, 1989c.
- Snazelle, Bancroft & Ullstrup, Phytopathology 61:1059, 1971.
- Teakle & Grylls, Aust. J. agric. Res. 24: 465, 1973.
- Teakle, Shukla & Ford, AAB Descr. Pl. Viruses 342, 5 pp., 1989.
- Thongmeearkom, Ford & Jedlinski, Phytopathology 66: 332, 1976.
- Tosic & Ford, Phytopathology 62: 1466, 1972.
- Tosic & Ford, Phytopathology 64: 312, 1974.
- Tosic & Ford, Proc. int. Maize Virus Dis. Colloq. Workshop, Wooster, Ohio, 1982: 229, 1983.
- Tosic & Sutic, Annls Phytopath. 9: 403, 1977.
- Tosic, Ford, Moline & Mayhew, Phytopathology 64: 439, 1974.
- Tosic, Ford, Shukla & Jilka, Pl. Dis. 74: 549, 1990.
- Tu & Ford, Phytopathology 61: 1516, 1971.
- Von Baumgarten & Ford, Phytopathology 71: 36, 1981.
- Williams & Alexander, Phytopathology 55: 802, 1965.
- Williams, Findley, Dollinger & Ritter, Pl. Dis. Reptr 52: 863, 1968.