Details of DPV and References
DPV NO: 351 December 1989
Family: Virgaviridae
Genus: Tobamovirus
Species: Tobacco mild green mosaic virus | Acronym: TMGMV
Tobacco mild green mosaic virus
C. Wetter Department of Botany, University of the Saarland D-66, Saarbrücken, Germany
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Disease described by McKinney (1929).
- Synonyms
- Mild dark-green tobacco mosaic virus (Rev. appl. Mycol. 9: 260;
18: 674)
- Para-tobacco mosaic virus (Rev. appl. Mycol. 27: 262)
- Mild strain of tobacco mosaic virus (Johnson, 1947)
- South Carolina mild mottling strain of tobacco mosaic virus (McKinney, 1952)
- Strains U2 and U5 of tobacco mosaic virus (Rev. appl. Mycol. 34: 110; 40: 383)
- Green-tomato atypical mosaic virus (Rev. appl. Mycol. 41: 674)
- Para-tobacco mosaic virus (Rev. appl. Mycol. 27: 262)
-
A virus with RNA-containing tubular rod-shaped particles c. 308 x 18 nm, easily transmitted by mechanical inoculation and by handling during cultivation. No natural vector known. Widely distributed throughout the world. Of economic importance for tobacco crops.
Main Diseases
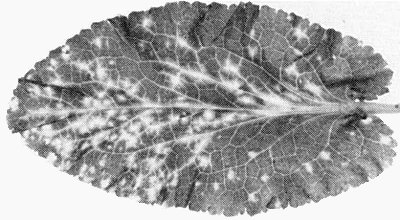
Bright yellow mosaic symptoms occur in Nicotiana glauca plants naturally infected by the U5 type virus (McKinney, 1929; Bald & Goodchild, 1960) (Fig. 1). Mild green mosaic symptoms with oak leaf patterns occur in naturally infected Turkish tobacco cv. Samsun and cultivars of Burley tobacco grown in West Germany (Fig. 2). Most cultivars of pepper react with severe mosaic symptoms (Fig. 4) and necrosis followed by leaf drop (Wetter, 1984a). The symptoms induced in cultivated gesneriads are inconspicuous (Zettler & Nagel, 1983).
Geographical Distribution
Occurs probably in all tropical and subtropical regions where Nicotiana glauca is distributed: North America (Bald & Goodchild, 1960), Australia (Randles et al., 1981) and many European and African countries including Madeira, the Mediterranean and Canary Islands (McKinney, 1929; Wetter, 1984b). The virus occurs much more frequently than type tobacco mosaic virus in field tobacco in West Germany (Köhler & Panjan, 1943; Wetter, 1980) and was found in field tobacco also in Wisconsin, USA (Weber, 1951). Cigarettes with light tobacco may contain both of these tobamoviruses and may play a role in their infection cycle (Wetter, 1980; Wetter & Bernard, 1977). The virus was isolated from Brazilian cigarettes but not from Chinese cigarettes (Wetter, 1984b). Widespread in many species of cultivated gesneriads in USA (Zettler & Nagel, 1983). Found also in plants of the monocotyledonous plant Rhoeo spathacea in Florida, USA (Baker & Zettler, 1988).
Host Range and Symptomatology
No extensive host range studies have been made. The virus infects many solanaceous species and also infects species of the families Chenopodiaceae, Commelinaceae, Gesneriaceae and Umbelliferae. Causes mild mosaic or mottle symptoms in many cultivars of tobacco. In later stages of infection leaves show weak or no symptoms. Late systemic infection of Eryngium planum is symptomless. Rhoeo spathacea shows conspicuous mosaic symptoms (Baker & Zettler, 1988).
-
Diagnostic species
- Chenopodium quinoa.
Chlorotic local lesions; no systemic infection. - Datura stramonium. Small necrotic local lesions; no systemic infection.
- Eryngium aquaticum, E. planum. Systemic yellow flecks (Fig. 3) followed by symptomless infection of leaves produced subsequently. Not infectible with type tobacco mosaic virus or tomato mosaic virus.
- Lycopersicon esculentum. No infection.
- Nicotiana debneyi, N. clevelandii. Symptomless local infection; systemic mosaic.
- Nicotiana glutinosa, N. sylvestris, N. tabacum cvs White Burley and Xanthi-nc. Small necrotic local lesions; no systemic infection.
- Eryngium aquaticum, E. planum. Systemic yellow flecks (Fig. 3) followed by symptomless infection of leaves produced subsequently. Not infectible with type tobacco mosaic virus or tomato mosaic virus.
-
Propagation species
- Nicotiana clevelandii, N. debneyi
or N. tabacum cv. Samsun may be used for propagation. Eryngium planum is the best host for maintaining pure virus cultures.Assay species
- Datura stramonium, Nicotiana glutinosa, N. sylvestris
and N. tabacum cvs Xanthi-nc, White Burley (Fig. 5). The production of necrotic lesions by tobacco mild green mosaic virus in N. sylvestris is reduced up to 90% by an excess of type tobacco mosaic virus in the inoculum (Siegel, 1959). - Altschuh, Reinbolt & Van Regenmortel, J. gen. Virol. 52: 363, 1981.
- Atreya & Siegel, Virology 168: 388, 1989.
- Baker & Zettler, Pl. Dis. 72: 513, 1988.
- Bald & Goodchild, Phytopathology 50: 497, 1960.
- Betto, Bassi, Favali & Conti, Phytopath. Z. 75: 193, 1972.
- Boedtker & Simmons, J. Am. Chem. Soc. 80: 2550, 1958.
- Cohen, Siegel, Zaitlin, Hudson & Wildman, Phytopathology 47: 694, 1957.
- Dünnebier, Thesis, Department of Physics, Univ. Saarbrücken 1988.
- Ernwein, Thesis, Department of Botany, Univ. Saarbrücken 1985.
- Fassell & Wildman, Biochim. biophys. Acta 72: 230, 1963.
- Gibbs, CMI/AAB Descr. Pl. Viruses 184, pp. 6, 1977.
- Ginoza & Atkinson, Virology 1: 253, 1955.
- Ginoza, Atkinson & Wildman, Science, N. Y. 119: 269, 1954.
- Granett & Shalla, Phytopathology 60: 419, 1970.
- Helms, Virology 27: 346, 1965.
- Holmes & Franklin, Virology 6: 328, 1958.
- Johnson, Phytopathology 37: 822, 1947.
- Knight, Silva, Dahl & Tsugita, Virology 16: 236, 1962.
- Köhler & Panjan, Ber. dt. hot. Ges. 61: 175, 1943.
- Kreibig & Wetter, Z. Naturf., Ser. B 35c: 750, 1980.
- McKinney, J. agric. Res. 39: 557, 1929.
- McKinney, J. agric. Res. 51: 951, 1935.
- McKinney, Pl. Dis. Reptr 36: 184, 1952.
- Mundry & Priess, Virology 46: 86, 1971.
- Palukaitis & Symons, Virology 107: 354, 1980.
- Randles, Search 2: 30, 1971.
- Randles, Palukaitis & Davies, Ann. appl. Biol. 98: 109, 1981.
- Rentschler, Mol. gen. Genet. 100: 84, 1967.
- Rochon, Kelly & Siegel, Virology 150: 140, 1986.
- Shalla, Virology 35: 194, 1968.
- Shalla, Petersen & Giunchedi, Virology 66: 105, 1975.
- Siegel, Virology 8: 470, 1959.
- Siegel, Virology 46: 50, 1971.
- Siegel & Hudson, Biochim. biophys. Acta 34: 254, 1959.
- Siegel & Norman, Virology 6: 725, 1958.
- Siegel & Wildman, Phytopathology 44: 277, 1954.
- Siegel & Wildman, Virology 2: 69, 1956.
- Siegel, Wildman & Ginoza, Nature, Lond. 178: 1117, 1956.
- Siegel, Ginoza & Wildman, Virology 3: 554, 1957.
- Singer, Bald, Wildman & Owen, Science N.Y. 114: 463, 1951.
- Skotnicki, Scotti & Gibbs, Intervirology 7: 292, 1976.
- Solberg & Bald, Virology 21: 300, 1963.
- Streeter & Gordon, Phytopathology 56: 419, 1966.
- Tsugita, J. molec. Biol. 5: 293, 1962.
- Valverde & Dodds, J. gen. Virol. 67: 1875, 1986.
- Valverde & Dodds, J. gen. Virol. 68: 965, 1987.
- Van de Walle & Siegel, Virology 73: 413, 1976.
- Van de Walle & Siegel, Phytopathology 72: 390, 1982.
- Van Regenmortel, Virology 31: 467, 1967.
- Weber, Phytopathology 41: 593, 1951.
- Wetter, Z. PflKrankh. PflPath. PflSchutz 87: 150, 1980.
- Wetter, Pl. Dis. 68: 597, 1984a.
- Wetter, Phytopathology 74: 1308, 1984b.
- Wetter, in The Plant Viruses, Vol. 2: p. 205, eds M. H. V. Van Regenmortel & H. Fraenkel-Conrat, 424 pp., New York: Plenum Press, 1986.
- Wetter & Altschuh, J. Phytopath. 119: 160, 1987.
- Wetter & Bernard, Phytopath. Z. 90: 257, 1977.
- Wetter, Dore & Bernard, J. Phytopath. 119: 333, 1987.
- Wittmann, Z. Naturf., Ser. B 20b: 1213, 1965.
- Zettler & Nagel, Pl. Dis. 67: 1123, 1983.
- Zrein, Burckard & Van Regenmortel, J. virol. Methods 13: 121, 1986.
Strains
Minor variants differ in their ability to induce yellow or green symptoms in N. glauca (Bald & Goodchild, 1960). Different isolates from different hosts are not as variable as type tobacco mosaic virus in symptom expression in Turkish tobacco cv. Samsun. In Turkish tobacco no yellow spots occur as a result of spontaneous mutation, as happens with type tobacco mosaic virus (McKinney, 1935; Köhler & Panjan, 1943).
Transmission by Vectors
No vector is known, but spread of the virus in stands of N. glauca by an unknown vector is assumed (Randles et al., 1981). Spread along the roadsides where N. glauca is planted may result from mechanical transmission.
Transmission through Seed
Not transmissible through seed of Nicotiana glauca (Randles et al., 1981).
Serology
The virus is a good immunogen. Intravenous and/or intramuscular injections of a total of 10 mg virus with Freund’s incomplete adjuvant gave antiserum titres up to 1/16,400 in slide precipitin tests. Double antibody sandwich ELISA can be used to detect closely related isolates and indirect ELISA is suitable for determinations of distant relationships (Wetter et al., 1987). Indirect ELISA in combination with the biotin-avidin system (Zrein et al., 1986) is the most sensitive method for determining antigen and antibody titres. No differences have been detected in immunodiffusion tests between strains/isolates of the virus from different hosts from widely separated locations of the world (Fig. 6), with one exception: isolate It III from pepper in Italy could be distinguished by antisera to several strains of tobacco mild green mosaic virus but antisera to It III were not able to differentiate this strain from the others (Wetter & Altschuh, 1987). All antisera tested so far contain antibodies to normal host proteins when tested in indirect ELISA (C. Wetter, unpublished data).
Relationships
Tobacco mild green mosaic virus is a characteristic member of the tobamovirus group. Serological differentiation indices between tobacco mild green mosaic, tobacco mosaic and tomato mosaic viruses correlate with the degree of difference in the amino acid sequence of their coat proteins. The SDI values between these viruses in reciprocal tests were as follows. Tobacco mosaic virus/tomato mosaic virus: 2; tobacco mosaic virus/tobacco mild green mosaic virus: 2.5; tomato mosaic virus/tobacco mild green mosaic virus: 3 (Wetter, 1984b). In indirect ELISA serological relationships have also been demonstrated to the following other members of the tobamovirus group: odontoglossum ringspot, bell pepper mottle, pepper mild mottle, ribgrass mosaic, frangipani mosaic, Sammons’ opuntia, sunn-hemp mosaic, and cucumber green mottle mosaic (cucumber virus 4) viruses (Wetter et al., 1987; C. Wetter, unpublished data) (Fig. 7). Various hybridization methods revealed a high percentage of nucleotide sequence homology between strains of the virus e.g. U2 and green-tomato atypical mosaic virus. No homology, or very little, was found between tobacco mild green mosaic virus and other members of the tobamovirus group such as type tobacco mosaic virus (Van de Walle & Siegel, 1976, 1982; Palukaitis & Symons, 1980; Randles et al., 1981).
Stability in Sap
The virus is less stable than type tobacco mosaic virus (Fassell & Wildman, 1963). In tobacco sap it lost infectivity after 10 min at 85°C or after dilution beyond 10-7 with distilled water. In herbarium specimens of N. glauca it was still infective after 45 years (Randles, 1971).
Purification
Readily purified by many procedures such as salt or isoelectric precipitation. The following method gives a good yield: inoculate fully expanded leaves of Samsun tobacco and harvest them 3 weeks later. Blend each 100 g fresh or frozen leaves in 100 ml 0.1 M phosphate buffer, pH 7.5, containing 0.2% (w/v) sodium sulphite, 0.2% (w/v) diethyldithiocarbamate, and 5 mM EDTA. Strain the extract through cheesecloth and clarify further by adding 0.2 vol of a mixture (1:1; v/v) of butan-1-ol and chloroform and stir for 15 min. Centrifuge at low speed and filter supernatant fluid through cotton wool. Precipitate the virus by adding polyethylene glycol (M. Wt 6000) to 4% (w/v) and NaCl to 4% (w/v). Centrifuge at low speed and resuspend the pellet in 0.01 M phosphate buffer, pH 7.5. Purify further by two or more cycles of differential centrifugation. Yields may reach > 1 g/kg tissue but are usually lower. Virus-containing sediments after ultracentrifugation are colourless. The virus does not absorb green or brown host substances as does tobacco mosaic virus (Siegel & Wildman, 1954; Ginoza et al., 1954). Substantially monodisperse preparations made according to the method of Boedtker & Simmons (1958) form brilliant colloidal crystals in vitro which may be stable for some years (Kreibig & Wetter, 1980; Dünnebier, 1988). Separation of a mixture of the virus with type tobacco mosaic virus in purified preparations is possible on a large scale by continuous free-flow electrophoresis (Streeter & Gordon, 1966).
Properties of Particles
Rate zonal centrifugation of purified virus or of clarified sap in sucrose produces a single band containing particles of uniform length.
Sedimentation coefficient (s20, w) at infinite dilution: 186 S (Wetter, 1986).
Isoelectric point: pH 4.17 (Ginoza & Atkinson, 1955).
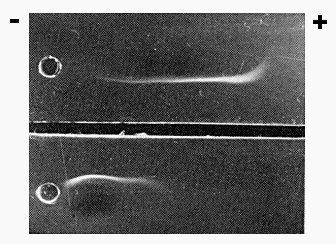
Electrophoretic mobility: -4.9 x 10-5 cm2/sec/V at pH 6.9 in 0.1 M ionic strength cacodylate buffer (Singer et al., 1951; Siegel & Wildman, 1954) (Fig. 11).
Extinction coefficient (A0.1%, 1cm) at 260 nm uncorrected for light-scattering: 3.16 (Wetter, 1986).
A260/A280: 1.22; A260(max)/A248(min): 1.1 (Wetter, 1986).
Buoyant density in CsCl: 1.307 (Siegel & Hudson, 1959); 1.322 (Skotnicki et al., 1976).
Inactivation by ultraviolet irradiation: About 5.5 times more sensitive than type tobacco mosaic virus (Siegel & Wildman, 1956; Siegel et al., 1956). Inactivation at the wavelengths 254 nm and 280 nm is due to energy absorption by the nucleic acid moiety. At 226 nm energy is absorbed by the particle protein to the same extent as with the particle protein of type tobacco mosaic virus (Siegel & Norman, 1958).
Disassembly: The RNA is more easily released than that of tobacco mosaic virus by the heat-detergent method (Siegel et al., 1956).
Particle Structure
Rigid tubular rods, length c. 308 nm, max diameter c. 18 nm (Wetter, 1986). Composed of c. 2130 identical protein subunits around a cylindrical channel of radius 2 nm. The protein density at radius 2.4 nm is much less than that of tobacco mosaic virus. The RNA lies at a radius of 4 nm. The protein subunits are helically arranged, 49.05 in three turns; pitch c. 2.3 nm (Holmes & Franklin, 1958).
Particle Composition
Nucleic acid: Single-stranded RNA, M. Wt 2 x 106, c. 5% of particle weight (Gibbs, 1977). Nucleotide base ratios are: G 23.8; A 30.9; C 17.2; U 28.1 (Knight et al., 1962). The nucleotide sequence differs from that of type tobacco mosaic virus (Mundry & Priess, 1971). In preparations of the U2 strain 2-2.5% of the particles are ‘pseudovirions’ containing RNA molecules complementary to chloroplast DNA or to a lesser extent to nuclear DNA (Siegel, 1971).
Protein: About 95% of particle weight. Subunits, of M. Wt 17.5 x 103 each consist of a chain of 158 amino acids with the following sequence (Wittmann, 1965; Rentschler, 1967; Altschuh et al., 1981):
| 1 | 10 |
| Pro-Tyr-Thr-Ile-Asn-Ser-Pro-Ser-Gln- | Phe-Val-Tyr-Leu-Ser-Ser-Ala-Tyr-Ala-Asp |
| 20 | 30 |
| Pro-Val-Glu-Leu-Ile-Asn-Leu-Cys-Thr-Asn- | Ala-Leu-Gly-Asn-Gln-Phe-Gln-Thr-Gln-Gln |
| 40 | 50 |
| Ala-Arg-Thr-Thr-Val-Gln-Gln-Gln-Phe-Ala- | Asp-Ala-Trp-Lys-Pro-Ser-Pro-Val-Met-Thr |
| 60 | 70 |
| Val-Arg-Phe-Pro-Ala-Ser-Asp-Phe-Tyr-Val- | Tyr-Arg-Tyr-Asn-Ser-Thr-Leu-Asp-Pro-Leu |
| 80 | 90 |
| Ile-Thr-Ala-Leu-Leu-Asn-Ser-Phe-Asp-Thr- | Arg-Asn-Arg-Ile-Ile-Glu-Val-Asn-Asn-Glu |
| 100 | 110 |
| Pro-Ala-Pro-Asn-Thr-Thr-Glu-Ile-Val-Asn- | A1a-Thr-G1n-Arg-Va1-Asp-Asp-Ala-Thr-Val |
| 120 | 130 |
| Ala-Ile-Arg-Ala-Ser-Ile-Asn-Asn-Leu-Ala- | Asn-Glu-Leu-Val-Arg-Gly-Thr-Gly-Met-Phe |
| 140 | 150 158 |
| Asn-Gln-Ala-Gly-Phe-G1u-Thr-Ala-Ser-Gly- | Leu-Val-Trp-Thr-Thr-Thr-Pro-Ala-Thr |
Few amino acid exchanges have been found in naturally occurring strains or in chemically evoked mutants (Van Regenmortel, 1967; Tsugita, 1962; Wetter & Altschuh, 1987).
Satellite
The U5 strain from native Nicotiana glauca in California is a helper virus for the synthesis of a satellite virus. This has a ssRNA (M. Wt 0.38 x 106) which is encapsidated in isometric particles with a diameter of 17 nm. The capsid protein (M. Wt 18 x 103) of the satellite is not related serologically to the protein of the helper virus nor to the proteins of satellite tobacco necrosis virus or satellite panicum mosaic virus. The replicative dsRNA of the satellite (M. Wt 0.6 x 106) is more abundant than the replicative dsRNA (M. Wt 4.3 x 106) of the virus (Valverde & Dodds, 1986). Hybridization experiments with cDNA showed no homology between satellite RNA and helper RNA. The satellite virus is systemically distributed throughout the plant in high concentration along with the helper virus. It does not influence the symptoms caused by the helper virus. Type tobacco mosaic virus can also serve as a helper but less efficiently (Valverde & Dodds, 1987).
Relations with Cells and Tissues
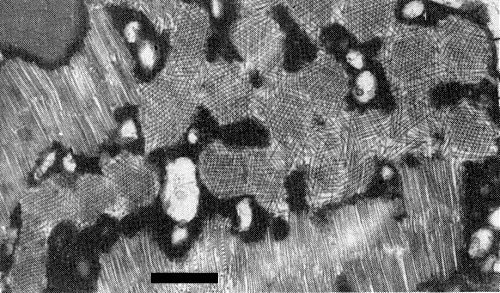
Many kinds of cell are infected e.g. hair, epidermal, mesophyll and phloem cells of Samsun tobacco, Nicotiana debneyi and Eryngium planum (Ernwein, 1985). The bulk of the particles accumulate in the cytoplasm and form crystalline multilayer or angled-layer aggregates (Fig. 10) (Granett & Shalla, 1970). No X-bodies, or very few, were observed (Shalla, 1968; Ernwein, 1985). Virus particles occur in most chloroplasts, but were found to be shorter than 300 nm and not infective (Shalla et al., 1975). Autoradiographic studies suggest that virus replication probably does not take place within chloroplasts (Betto et al., 1972). Virus particles occur also in intact and disintegrated nuclei (Ernwein, 1985; Wetter, 1986). Most electron microscope observations support the view that synthesis and assembly of the virus particles occur in the cytoplasm in association with membranes (Ernwein, 1985; Fig. 9). The ‘pseudovirions’ (see Particle Composition) in chloroplasts presumably arise by entry of cytoplasmically synthesized capsid protein into the chloroplasts where assembly takes place with transcripts of chloroplast DNA (Rochon et al., 1986), especially with a 18 S rRNA. This was found to contain three sites (loop structures) able to initiate assembly with the capsid protein of the U2 strain (Atreya & Siegel, 1989). The occurrence of pseudovirions in nuclei may be explained in a similar way.
Notes
Mixed infections of tobacco mild green mosaic virus and type tobacco mosaic virus occur frequently in Nicotiana glauca, cultivars of N. tabacum and species of gesneriads. To eliminate tobacco mild green mosaic virus from the mixture it is necessary to transfer the cultures repeatedly in N. sylvestris until strains producing necrotic lesions are eliminated. Tobacco mild green mosaic virus can be freed from type tobacco mosaic virus or tomato mosaic virus by passage through Eryngium planum. Several authors working with U2 and U5 strains reported Lycopersicon esculentum as a systemic host. This is quite unlikely because all cultivars of tomato tested so far have proved to be immune (Wetter, 1984b). It is more probable that tomato became infected because of contamination of the inoculum with type tobacco mosaic virus or tomato mosaic virus. Tobacco mild green mosaic virus differs from pepper mild mottle virus in infecting Capsicum frutescens locally only, whereas the latter virus infects this host systemically. Besides these host reactions serological tests are a reliable method of distinguishing tobacco mild green mosaic virus from the above mentioned and other tobamoviruses.
Much work has been done with U2 and U5 strains in order to elucidate phenomena such as competition, cross-protection and interference, including the apparent systemic transport of a resistance factor (Fig. 5), between this virus and type tobacco mosaic virus. Our understanding of these interactions in the host is still fragmentary (Wetter, 1986). The survival of tobacco mild green mosaic virus in mixed infections with the more stable and predominating type tobacco mosaic virus (Cohen et al., 1957) probably results from a special adaptation to competition in the host. The virus has a shorter lag phase (2.5 h) than does type tobacco mosaic virus (5 h) (Siegel et al., 1957), it invades the host plant tissue more rapidly (Helms, 1965), and moves closer to the apex (Solberg & Bald, 1963). The coexistence of the two viruses in plants has led in the past to difficulties in distinguishing and separating them.
Figures

Leaves of Nicotiana tabacum cv. White Burley showing systemic acquired resistance. Left: Leaf inoculated first on the apical half with 0.1 mg/ml virus and 7 days later with a challenge inoculum on the basal half. Right: Control leaf given only the second inoculation on the basal half.

Immunodiffusion reaction with tobacco mild green mosaic virus antiserum (central wells). (Fig. 6) Outer wells contain the following viruses: a = isolate from N. glauca, Gran Canaria; b = isolate from pepper, Italy; c = isolate from N. glauca, Corsica; d = original isolate of McKinney (1929); e = U2 strain; f = green-tomato atypical mosaic virus; g = Johnson’s mild strain; h = para-tobacco mosaic virus. (Fig. 7) Outer wells containing the following tobamoviruses: a = tobacco mild green mosaic; b = tomato mosaic; c = sunn-hemp mosaic; d = ribgrass mosaic; e = odontoglossum ringspot; f = Ohio III strain of tobacco mosaic; g = cucumber green mottle mosaic (cucumber virus 4); h = tobacco mosaic.

Immunodiffusion reaction with tobacco mild green mosaic virus antiserum (central wells). (Fig. 6) Outer wells contain the following viruses: a = isolate from N. glauca, Gran Canaria; b = isolate from pepper, Italy; c = isolate from N. glauca, Corsica; d = original isolate of McKinney (1929); e = U2 strain; f = green-tomato atypical mosaic virus; g = Johnson’s mild strain; h = para-tobacco mosaic virus. (Fig. 7) Outer wells containing the following tobamoviruses: a = tobacco mild green mosaic; b = tomato mosaic; c = sunn-hemp mosaic; d = ribgrass mosaic; e = odontoglossum ringspot; f = Ohio III strain of tobacco mosaic; g = cucumber green mottle mosaic (cucumber virus 4); h = tobacco mosaic.

Purified virus particles from cigarette tobacco containing para-tobacco mosaic strain. Bar represents 100 nm (courtesy: R. G. Milne).
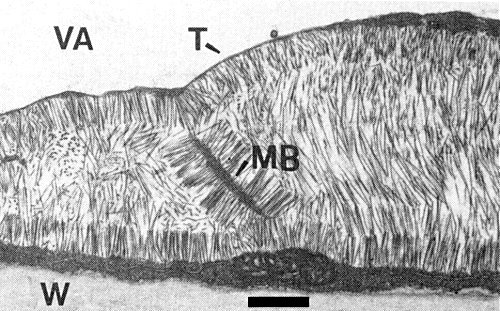
Virus inclusion in an epidermal cell of Samsun tobacco. Note the attachment of virus particles to the tonoplast (T) and to membrane structures (MB); VA = vacuole; W = cell wall. Bar represents 600 nm (Ernwein, 1985).