Details of DPV and References
DPV NO: 364 September 1998
Family: Secoviridae
Genus: Nepovirus
Species: Peach rosette mosaic virus | Acronym: PRMV
This is a revised version of DPV 150
Peach rosette mosaic virus
D. C. Ramsdell Department of Botany and Plant Pathology, Michigan State University, East Lansing, MI 48824, USA
J. M. Gillett Department of Botany and Plant Pathology, Michigan State University, East Lansing, MI 48824, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by
Cation (1933,
1942);
Dias (1972,
1975);
Dias & Cation (1976),
Dias & Allen (1980);
Ramsdell & Myers (1974),
Selected synonyms
- Rosette mosaic virus (Klos, 1976)
- Grape decline virus (Dias, 1972)
- Grapevine degeneration virus (Rev. appl. Mycol. 54: 1375)
- Grape decline virus (Dias, 1972)
A virus with isometric particles about 28 nm in diameter sedimenting as three components and containing two genomic species of single-stranded RNA. In nature, it is transmitted by the nematode Xiphinema americanum sensu lato and by one population of Longidorus diadecturus.
Main Diseases
Peach rosette mosaic virus causes diseases in grapevine, peach and blueberry. In grapevine (Vitis labrusca cv. Concord and others) it induces delayed dormancy breaking, late and uneven bloom, small and uneven berry clusters (Fig. 3), leaf deformity and mottling, and cane growth is short and crooked (Fig. 1) giving vines an umbrella-like growth habit (Fig. 2). Vines become unproductive and may die (Ramsdell & Myers, 1978). Ramsdell & Gillett (1985) and Ramsdell, Gillett & Bird (1995) have published a partial list of resistant and susceptible vine cultivars. In peach, it causes a rosette mosaic disease (Prunus persica) in which trees show delayed foliation, chlorotic mottling, and distortion to early formed leaves and shortening of the internodes to produce a rosetted appearance (Fig. 4, Fig. 5). In highbush blueberry (Vaccinium corymbosum cvs Jersey and Berkley) it causes leaves to become strap-like or crescent shaped (Fig. 6). Symptoms are not uniformly distributed over the entire bush.
Geographical Distribution
Found regularly in Michigan, USA (Dias, 1975, Dias & Cation, 1976), once in New York State, USA (Hildebrand, 1941) and occasionally in Ontario, Canada (Allen et al., 1982).
Host Range and Symptomatology
Infects Concord grapevine and peach in commercial plantings. The virus also
infects some weeds, e.g. Taraxacum officinale (dandelion),
Solanum carolinense (Carolina horsenettle) and Rumex crispus
(curly dock)
(Ramsdell & Myers, 1978).
The experimental herbaceous host
range is rather narrow. Some species of Chenopodiaceae, Cucurbitaceae,
Fabaceae and Solanaceae can be infected by mechanical inoculation with
sap.
Diagnostic species
- Chenopodium amaranticolor. Faint chlorotic lesions develop in
inoculated leaves within 7-14 days. Systemic symptoms may include mottling
and leaf deformity, distortion and dieback of the shoot tip.
Overall stunting of the plant results.
- C. quinoa. Cream to yellow chlorotic lesions develop in inoculated leaves within 7-14 days. Systemic mottling, leaf deformity, and epinasty occur a few days after local lesions appear. Apical necrosis may also occur.
- Nicotiana tabacum cv. Harrow Velvet. Chlorotic lesions or necrotic ringspots are induced in inoculated leaves but only by virulent strains; mild but transient chlorotic ringspots occur later.
- Serological methods should always be used for identification of the virus.
- C. quinoa. Cream to yellow chlorotic lesions develop in inoculated leaves within 7-14 days. Systemic mottling, leaf deformity, and epinasty occur a few days after local lesions appear. Apical necrosis may also occur.
Propagation species
- Chenopodium amaranticolor and C. quinoa are both good hosts
for
maintaining cultures. C. quinoa is the best source of virus for
purification.
Assay species
- No reliable local lesion host has been reported, but in autumn and winter (cool season) and with virulent isolates, C. quinoa sometimes may be useful, especially if the leaves are soft and succulent.
Strains
Nearly all isolates from peach and grapevine produce reactions of complete identity in gel double-diffusion tests with homologous or heterologous antisera, and share all antigenic groups with each other in cross-absorption tests. In one instance, however, a less virulent isolate from grapevine contained additional antigenic determinants not present in other isolates from peach or grapevine as detected by cross-absorption and spur formation in gel double-diffusion tests (Dias, 1972; Dias & Cation, 1976).
Transmission by Vectors
The virus is soil-borne; healthy peach trees and grapevines become infected when planted in contaminated soils (Cation, 1942, 1951). Steam treatment of infested soil from peach orchards prevented transmission (Fulton & Cation, 1959). Two nematode species, Xiphinema americanum and Criconemoides spp., were reported to be vectors (Klos et al., 1967), but the record for Criconemoides needs confirmation. As a result of work by Lamberti & Bleve-Zacheo (1979) and others, X. americanum has now been split into a current total of 44 species of which 21 are present in North America and 12 are regarded as indigenous (Robbins & Brown, 1991; Robbins, 1993). Consequently all earlier reports of virus transmission must now be referred to as X. americanum sensu lato. Ramsdell & Myers (1974) found large populations of X. americanum sensu lato and Criconemoides xenoplax and on occasions, low populations of Trichodorus spp. in soils from infested vineyards. Under glasshouse conditions, hand-picked X. americanum sensu lato transmitted the virus from C. quinoa, infected mechanically, to healthy C. quinoa, but not to healthy grapevine; percentage transmission was low and erratic (H. F. Dias, unpublished data). Longidorus diadecturus was identified morphometrically and reported as a vector-nematode associated with PRMV-diseased peach in southwestern Ontario, Canada, but no actual nematode transmission data were reported (Dias & Cation, 1976; Dias & Allen, 1980). Allen et al. (1982) demonstrated transmission of virus from C. quinoa to peach and grape using viruliferous Longidorus diadecturus washed from infested peach soil. Larvae appeared to be more efficient vectors than adults. Allen et al. (1984) reported that L. diadecturus was a slightly more efficient vector than X. Americanum sensu lato in tests with hand-picked nematodes taken from roots of cucumber plants that had been grown in Ontario soils from around infected peach trees. Allen (1986) showed in comparative tests using herbaceous plants, that L. diadecturus was an efficient vector but that L. breviannulatus was not (only 1/52 test plants became infected). The aphid Myzus persicae failed to transmit the virus from C. quinoa to C. quinoa, irrespective of the length of the acquisition feeding period (Dias & Cation, 1976).
Transmission through Seed
Transmitted to about 9.5% of the seed of grapevine cv. Concord
(Ramsdell & Myers, 1978).
Also seed-borne in dandelion
(Ramsdell & Myers, 1978)
and C. quinoa
(Dias & Cation, 1976).
Transmission by Dodder
Not transmitted by Cuscuta campestris (Dias & Cation, 1976).
Serology
The virus is moderately immunogenic. Antiserum titres of 1/512 to 1/1024 can be obtained by intramuscular injection of purified virus in Freund’s complete adjuvant for the first injection, followed by virus in Freund’s incomplete adjuvant for two additional injections. The virus forms a single precipitin line in gel-double diffusion serological tests.
Relationships
The virus did not react with antisera to blueberry leaf mottle, grapevine fanleaf, grapevine chrome mosaic, prune dwarf, raspberry ringspot, tomato ringspot, tobacco ringspot, tomato black ring, tomato bushy stunt, or tomato top necrosis viruses or to several strains (type, elderberry, rhubarb and dogwood) of cherry leaf roll virus. ELISA works reasonably well for detection in blueberry and grapevine tissue (Ramsdell et al., 1979; Ramsdell & Gillett, 1981).
Stability in Sap
In C. quinoa sap, the thermal inactivation point (10 min) is 58-68°C, the dilution end point is 10-3-10-5 and the virus retains infectivity for about 15-25 days at room temperature (20°C). Sap retained infectivity after 3 months at -15°C (Dias & Cation, 1976).
Purification
The following method gives good yields of purified virus (1 to 3 mg/100 g starting
material)
(Dias & Cation, 1976).
All steps should be done at 0-4°C.
Blend 100 g infected symptom-bearing C. quinoa leaves (10 to 15 days
post-
inoculation) in 150 ml 0.5 M boric acid buffer adjusted to pH 6.5 with NaOH and
containing 0.5% (w/v) ascorbic acid. Filter extract through cheesecloth, then add
chloroform to 8.5% (v/v) and blend for 3 min. Centrifuge at 12,000 g for
15 min. To the supernatant fluid add 1 N HCl dropwise with stirring until the pH
becomes 5.3. Allow to stand for 30 min. Centrifuge at 12,000 g for 15
min. Ultracentrifuge the supernatant fluid at 105,000 g for 2.5 h.
Resuspend the pellets in 3 to 4 ml 0.01 M phosphate buffer, pH 7.0, overnight,
then centrifuge at 12,000 g for 5 min. Layer on to linear or linear-log 5-
30% (w/v) sucrose gradients in 0.01 M phosphate buffer, pH 7.0, and centrifuge
in a Beckman SW 41 rotor for 90 min at 38,000 rev./min or in a Beckman SW
25.1 rotor for 2 h at 23,000 rev./min. Collect M and B bands (the upper (T) band
is usually very faint).
Properties of Particles
The virus particles sediment as three components (T, M and B) in sucrose density gradients (Dias, 1975; Dias & Cation, 1976).
Sedimentation
coefficients, s20,w (svedbergs): 52 (T), 115 (M), 134 (B).
The top component (T) contains empty and disrupted particles; the middle (M)
and bottom (B) components contain intact virus particles.
Isoelectric point: pH 4.0 to 5.0.
A0.1%260nm, 1 cm (unfractionated
virus) assumed to be 10.
A260/A280 (unfractionated virus): 1.7 to 1.9 (not
corrected for light scattering).
Buoyant density in CsCl (g cm-3): 1.47 (M); 1.51 (B) (Dias & Cation, 1976).
Particle Structure
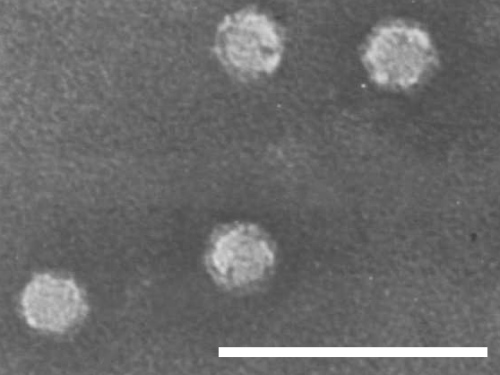
Isometric particles 28 nm in diameter (Fig. 7). (Dias, 1975; Ramsdell & Myers, 1974). Some particles are penetrated by negative stain.
Particle Composition
Nucleic Acid: single-stranded RNA. Two species, of Mr
2.2 x 106 and 2.5 x 106, determined under
denaturing conditions using 4% (v/v) formamide in 2.4% acrylamide gels
(Allen & Dias, 1977),
are found in M and B components, respectively. RNA-1 has
Tm values of 46, 58 and 70°C, respectively in 0.01 M
potassium phosphate buffer, pH 7.0, alone, or with 0.1 M NaCl or 1.0 M NaCl
added. Corresponding Tm values for RNA-2 were 47, 58 and
70°C, respectively. The temperature melting range was from 25 to
75°C
(Dias & Cation, 1976).
The mean nucleotide composition of
unfractionated RNA in mole percent was (G:A:C:U) 23.7 : 25.2 : 20.9 : 30.4. For
fractionated RNA, the corresponding values were 23.6 : 24.1 : 21.3 : 30.9 (RNA-
1) and 23.9 : 25.5 : 19.9 : 30.9 (RNA-2). Significant differences (P = 0.05)
between RNA-1 and RNA-2 were found only in the cytidylic acid content
(Dias & Cation, 1976;
Lammers et al., unpublished).
Protein: Subunits have an Mr of c. 57,000, estimated by electrophoresis in 10% SDS-polyacrylamide gels (Dias & Cation, 1976; Allen & Dias, 1977).
Relations with Cells and Tissues
In diseased grapevines, the virus is present in leaves, roots and fruit throughout the vine, although distribution of virus within the tissues may be uneven (D. C. Ramsdell, unpublished data). No information is available at the cellular or sub- cellular level.
Notes
Superimposed shallow and deep soil fumigation using shank injection of liquid or gaseous fumigants at high rates gives excellent prevention of re-infection of grapevines planted in soil previously infective (Ramsdell et al., 1983).
Figures
References list for DPV: Peach rosette mosaic virus (364)
- Allen, Can. J. Pl. Path. 8: 49, 1986.
- Allen & Dias, Can. J. Bot. 55: 1028, 1977.
- Allen, Van Schagen & Eveleigh, Can. J. Pl. Path. 4: 16, 1982.
- Allen, Van Schagen & Ebsary, Can. J. Pl. Path. 6: 29, 1984.
- Cation, Q. Bull. Mich. St. Univ. agric. Exp. Stn 16: 79, 1933.
- Cation, Tech. Bull. Mich. St. Univ. agric. Exp. Stn No. 180: 24 pp, 1942.
- Cation, U.S. Dep. Agric. Handb. No. 10: 14, 1951.
- Dias, Annls Phytopath. Numéro hors-série, 97, 1972.
- Dias, CMI/AAB Descr. Pl. Viruses 150, 1975.
- Dias & Allen, Can. J. Bot. 58: 1747, 1980.
- Dias & Cation, Can. J. Bot. 54: 1228, 1976.
- Fulton & Cation, Pl. Dis. Reptr 46: 991, 1959.
- Hildebrand, Phytopathology 31: 353, 1941.
- Klos, U.S. Dep. Agric. Handb. No. 437: 135, 1976.
- Klos, Fronek, Knierem & Cation, Q. Bull. Mich. St. Univ. agric. Exp. Stn 49: 287, 1967.
- Lamberti & Bleve-Zacheo, Nematol. Mediterranea 7: 51, 1979.
- Lammers, Allison & Ramsdell, J. gen Virol. (submitted), 1998.
- Ramsdell & Gillett, Pl. Dis. 65: 757, 1981.
- Ramsdell & Gillett, Phytopath. Mediterranea 24: 41, 1985.
- Ramsdell & Myers, Phytopathology 64: 1174. 1974.
- Ramsdell & Myers, Phytopathology 68: 447, 1978.
- Ramsdell, Gillett & Bird, Pl. Dis. 79: 154, 1995.
- Ramsdell, Andrews, Gillett & Morris, Pl. Dis. Reptr 63: 74, 1979.
- Ramsdell, Bird, Gillett & Rose, Pl. Dis. 67: 625, 1983.
- Robbins, J. Nematol. 25: 344, 1993.
- Robbins & Brown, Nematologica 37: 395, 1991.