Details of DPV and References
DPV NO: 370 March 2000
Family: Virgaviridae
Genus: Tobamovirus
Species: Tobacco mosaic virus | Acronym: TMV
This is a revised version of DPV 151
Tobacco mosaic virus
Milton Zaitlin Department of Plant Pathology, Cornell University, Ithaca, New York 14853, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Type member of the genus Tobamovirus. Disease first described in detail and transmitted experimentally by Mayer in 1886. Virus concept developed from studies of the disease by Beijerinck (1898).
Synonyms:
Common strain, wild type, vulgare
U1 (Siegel and Wildman, 1954)
OM, Japanese common strain (Nozu & Okada, 1968;
Watanabe, et al., 1999)
Korean common strain (Koh et al., 1992)
Brief description:
TMV is a rod-shaped virus, containing a single-stranded RNA molecule of sense polarity. The virus causes diseases in a broad variety of plant species. It has no known true vectors, but on rare occasions it is transmitted inefficiently by chewing insects. It is normally transmitted mechanically. It is best known for the contributions to virology which have been achieved through studies with TMV (Harrison & Wilson, 1999; Scholthof et al., 1999)
Main Diseases
Exhibits disease in many plant species (Holmes, 1939), although it is not always clear whether it is the type strain or other tobamoviruses which are described. In tobacco the virus elicits a "...classical mosaic..but may also cause veinbanding and is associated with a necrotic symptom known as mosaic burn" (Shew & Lucas, 1991).
Geographical Distribution
Worldwide
Host Range and Symptomatology
Host range given in Holmes (1946). The virus is known to infect at least 199 species from 30 plant families (Shew & Lucas, 1991). Subliminal infections seen in many species (Cheo & Gerard, 1971; Sulzinski & Zaitlin, 1982).
- Diagnostic species
Nicotiana tabacum cvs. Turkish, Turkish Samsun, Samsun (Samsoun), White Burley, Burley and Xanthi. Vein clearing appears in young, systemically-invaded leaves, 3-4 days post inoculation, followed by a light green-dark green mosaic, often accompanied by distortion and blistering (Figure 1). Inoculated leaves exhibit no symptoms other than faint chlorotic lesions when the plant nitrogen supply is limited. Plants may be stunted if they are infected while young.
N. glutinosa, N.tabacum cvs. Samsun NN, Xanthi NN and Xanthi-nc, plants with the N genotype, and Chenopodium amaranticolor, C. quinoa, and Phaseolus vulgaris cv. Pinto, form necrotic lesions which develop at the infection sites (Figure 2), but without systemic symptoms below about 28o. A systemic necrotic disease can develop above that temperature, in particular when the temperature is subsequently lowered below 28o. N. clevelandii and N.benthamiana exhibit a local necrosis, followed by systemic necrosis and plant death.
- Propagation species
N. tabacum, cvs. Turkish, Turkish Samsun, Samsun or Xanthi (nn).
- Assay species
Local lesion assays are most frequently performed with N. glutinosa, N. tabacum cvs. Xanthi nc, Xanthi NN, Samsun NN, Phaseolus vulgaris cv Pinto, Chenopodium amaranticolor or C. quinoa.
Strains
Many isolates once considered to be strains of TMV (Siegel & Wildman, 1954), are now separate tobamoviruses in the classification accepted by the International Committee on the Taxonomy of Viruses, based on recent sequencing information. There are, however, isolates from Japan (OM, Watanabe et al., 1999) and from Korea (Koh et al., 1992) with very close sequence homology to the type strain, which allows one to consider them as TMV strains.
Transmission by Vectors
Virus transmitted principally by mechanical inoculation. Virus has no true vectors, although there have been reports of incidental transmission by chewing insects, most probably by mechanical means (Lojek & Orlob, 1969; Harris & Bradley, 1973). Soil-borne virus particles or fragments of infected tissue can serve as sources of infection via roots. Virus is very persistent on clothing and on glasshouse structures (Broadbent & Fletcher, 1963).
Transmission through Seed
Not transmissible via seed or pollen. However, virus is often present in the seed coat, and there are occasional reports, principally with tomato, of plants consequently becoming infected by wounding of the embryo during germination (Broadbent, 1965).
Transmission by Dodder
Transmitted, chiefly among tobaccos, by Cuscuta campestris, C. japonica and C. subinclusa, but the particular strains or tobamoviruses have not always been identified. Virus does not replicate within the dodder. Subject reviewed by Hosford, 1967.
Serology
Good immunogen; high titre polyclonal antiserum may be produced from either virions or dissociated coat protein. Virus detected by a wide range of tests, but most commonly by ELISA tests, involving direct or indirect methods. Subject reviewed by Van Regenmortel (1986). Monoclonal antibodies have been produced (Briand et al., 1982).
Relationships
There are few true strains of TMV; rather, most are distinct tobamoviruses. The relationships are established by sequence comparisons and by serological cross reactions to the virions themselves or to the coat proteins. Tobamoviruses with only limited sequence similarity to the type strain do cross-react serologically. A masked strain inducing very mild symptoms in tobacco has been selected by growing type strain-infected tomato plants at 34.6oC (Holmes, 1934). It is serologically identical to the type strain. Sequence analysis of the genome shows relatively few changes from the type strain; the changes which attenuate symptoms are present in the 126-kDa gene (Holt et al., 1990).
Stability in Sap
Very stable; preparations of "unpreserved plant juice" retained infectivity after 50 years (Silber & Burk, 1965), although a temperature-sensitive, nitrous acid-induced mutant is much less stable (Hariharasubramanian et al., 1970). Very heat stable; some infectivity is retained after 10 minute exposures at over 90o C. Dilutions of 106 of expressed tobacco sap can be infectious. Tissue may be preserved by freezing fresh leaves, or by freeze-drying tissue. Purified virus preparations may be preserved at 4o C for long periods, using a few drops of chloroform to inhibit microorganisms.
Purification
Because of its high titre and stability, and its large particle size, TMV can be purified by many procedures such as ultracentrifugation, exclusion chromatography, or salt, polyethylene glycol, isoelectric or solvent precipitation. A method which has been shown to remove host plant contaminants from the virus is given by Asselin & Zaitlin (1978). Virus may be purified from crude preparations by agarose gel electrophoresis (Asselin & Grenier, 1985). TMV has the capacity to bind a host nucleoprotein, which can be removed with chelating agents (Ginoza et al., 1954). Particles may be sorted according to length using columns of agar or agarose beads (Steere, 1963). Yields may reach 10 mg.g-1 fresh tissue, but 1-3 mg.g-1 is more common.
Properties of Particles
Sedimentation coefficient (s20w) at infinite dilution is ca. 194S (Harrington & Schachman, 1956). Buoyant density is 1.325 g/cm3 (in CsCl; Siegel & Hudson, 1959). Particle weight is 39.4 x 106 Da (Caspar, 1963). Diffusion coefficient (D20,w) is ca. 4.4 x 10-8 cm2.sec-1 (Schramm & Bergold, 1947). Isolectric point is ca. pH 3.5 (Fraenkel-Conrat & Narita, 1958). Partial specific volume is ca. 0.73 cm3.g-1 (Lauffer, 1944). Electrophoretic mobility at ionic strength 0.075 and pH 6.5-7.9 is ca. -0.83 x 10-4 cm2.sec-1.V-1 (Kramer & Wittmann, 1958). Extinction coefficient at 260nm, 1 mg.ml-1, 1 cm light path, ranges between 2.7 and 3.5 (Brakke, 1967); a value of 3.0 is commonly used. A260/A280 is ca. 1.19 (Paul, 1958).
Particle Structure
Straight, rigid tubules (Figure 3); length ca. 300 nm, max. radius ca. 9 nm, composed of ca. 2140 identical protein subunits closely packed in a helix (pitch ca. 2.3 nm, 16 1/3 subunits/turn; Figure 4) around a cylindrical canal of radius ca. 2 nm. One continuous single strand of RNA of 6395 nucleotides, follows the same helix (49 nucleotides/turn or 3/subunit) at a radius of ca. 4 nm, and is associated with the protein subunits near their inner surfaces (Caspar, 1963) [Figure 5]. Particles can be dissociated into constituent nucleic acid and coat protein and reconstituted into stable infectious virus particles (Fraenkel-Conrat & Williams, 1955).
Particle Composition
Nucleic acid: Single-stranded linear RNA of 6395 nucleotides (Goelet et al., 1982). Represents ca. 5% of particle weight. Several subgenomic mRNAs (but not the coat protein mRNA) become encapsidated, resulting in particles of less than full length (Beachy & Zaitlin, 1977). Preparation of infectious RNA is described by Mandeles & Bruening (1968).
Protein: About 95% of particle weight is coat protein, comprised of 2130 identical molecules of 158 amino acids each (Wittmann-Liebold & Wittmann, 1967). Amino terminus is acetylated.
Lipid: None
Other components: Virions can bind Ca++ and/or Mg++ (Durham & Henry, 1977), but calcium ion peaks were not seen in X-ray fiber diffraction studies (Namba et al., 1989). There is about one molecule of ubiquitin per virion, linked by an isopeptide bond to the coat protein (Dunigan et al., 1988).
Satellite: An unusual, spherical, RNA-containing satellite virus was isolated from plants infected with the tobamovirus, tobacco mild green mosaic virus. TMV will support the satellite virus (Valverde et al., 1991), but there are no reports of natural occurrence of the satellite virus in association with TMV.
Genome Properties
Sequence of nucleic acid is archived in GenBank as V01408; the two sequences have some differences in the 5' untranslated leader (Goelet et al., 1982). The validity of such differences has been questioned (Meshi et al., 1983). Sequence of the Korean common strain is archived as X68110. There is a m7G5'ppp5'Gp cap on the 5' end (Zimmern, 1975). The 3' terminus is a tRNA-like structure with five pseudoknots (Van Belkum et al., 1985); it accepts histidine (Oberg & Philipson, 1972). In addition to the genomic RNA, there are 3 subgenomic RNAs (Figure 6). The 126-kDa replicase protein and its readthrough of 183-kDa are translated from the genomic RNA (Bruening et al., 1976; Hunter et al., 1976); readthrough at an amber UAG stop codon is potentiated by two suppressor tRNAs from tobacco (Beier et al., 1984). The I2-RNA translates into the 30-kDa movement protein (Bruening et al., 1976), and the LMC RNA is the mRNA for the coat protein (Hunter et al., 1976). A third mRNA (I1-RNA) encodes a 54-kDa protein (Sulzinski et al., 1985), but no corresponding protein has been detected in vivo. The sequences (nt 5420-5546; Jonard et al., 1977) which represent the nucleation site for assembly of the virion are about 1 kb from the 3' end of the RNA, within the open reading frame for the 30-kDa protein (Zimmern, 1977). Upon entry into the cell, cytoplasmic ribosomes associate with the virion on the 5' end of the RNA, and remove coat protein subunits (Wilson, 1984; Figure 7). Infectious transcripts have been generated from cDNA (Dawson et al., 1986).
The purified replicase (Osman & Buck, 1996) is composed of the 126-kDa and the 183-kDa viral-encoded proteins and a plant protein related to the RNA-binding subunit of yeast eIF-3 (Osman and Buck, 1997). The 126-kDa protein has guanylyltransferase activity (Dunigan & Zaitlin, 1990). The 30-kDa movement protein is an early product in the replication process, but degrades as the replication progresses (Padgett et al., 1996).
The profile of dsRNAs is shown in Zelcer et al. (1981).
Relations with Cells and Tissues
Virus replicates in several types of cells including mesophyll, epidermis, root hairs and trichomes. Particles are mainly in the cytoplasm, but may associate with chloroplasts (Shalla et al., 1975) and with cell walls (Esau, 1968). Many virion-like particles within chloroplasts are shorter than full length virions and may be pseudovirions, which are chloroplast RNAs encapsidated in TMV coat protein (Siegel, 1971). Coat protein in chloroplasts associates with thylakoid membranes (Reinero & Beachy, 1986).
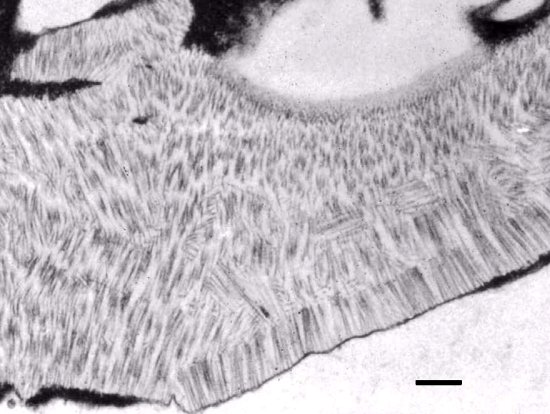
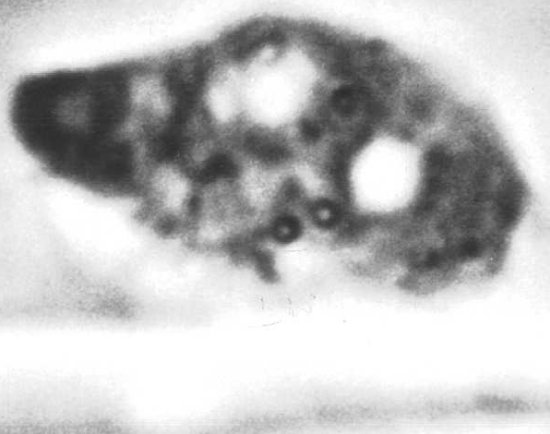
Cells contain crystalline inclusions composed of virus particles (Figures 8 & 9), and linear aggregates of needles, spindles and fibers. Amorphous inclusions, also called "X-bodies", are also present (Figure 10). Viral-induced replicase (Osman & Buck, 1996; 1997) and dsRNAs are associated with membranes; the 126-kDa replicase protein is found in the "X-bodies" (Figure 11; Hills et al., 1987).
Movement protein associates with plasmodesmata (Atkins et al., 1991), and with the microtubules (Figure 12) and actin filaments of the cytoskeleton (Heinlein et al., 1995). It modifies the size exclusion limit for molecules to transport through plasmodesmata (Wolf et al., 1989).
The infectious entity which transports the infection from cell to cell via plasmodesmata is RNA (Siegel et al., 1962). Virus particles (Saito et al., 1990; Simón-Buela & García-Arenal, 1999) are considered to be required for long distance movement in the host plant. The infectious entity is transported in the phloem (Bennett, 1939) but can pass through stem tissue without necessarily replicating there (Samuel, 1934). Invasion of minor veins does not require intact virions (Ding et al., 1996).
Ecology and Control
The virus is known wherever tobacco is grown, and is considered to be one of the most important tobacco viruses economically (Gooding, 1991). Insects are not important in its spread. Control is by crop rotation and effective sanitation; resistant cultivars of both flue-cured and burley tobacco are available (Gooding, 1991).
TMV has been used for the first demonstration of coat protein-mediated resistance (Powell Abel et al., 1986), replicase-mediated resistance (Golemboski et al., 1990) and movement protein-mediated resistance (Cooper et al., 1995).
Notes
The type strain used in many laboratories throughout the world apparently has a common origin. Personal recollections of C.A. Knight, W.C. Price, S.G. Wildman and F.O. Holmes suggest that the original isolate purified by W.M. Stanley (1935) came from James Johnson of the University of Wisconsin via L.O. Kunkel. The U1 isolate (Siegel & Wildman, 1954) and the German isolate 'vulgare' (Wittmann-Liebold & Wittmann, 1967) also came from Johnson. Japanese (Watanabe et al., 1999) and Korean (Koh et al., 1992) common strains apparently have independent origins.
An anthology reprinting 25 seminal articles (with commentaries), in which TMV studies have been shown to contribute basic knowledge to virology in general, and to plant virology specifically, is edited by Scholthof et al. (1999). A TMV historical volume commemorating the 100 year contribution of TMV studies, as presented in a symposium in Edinburgh in 1998, is edited by Harrison & Wilson (1999).
Figures

Local necrotic lesions produced upon TMV infection in Nicotiana tabacum of the NN genotype. Photographed one week post-inoculation.
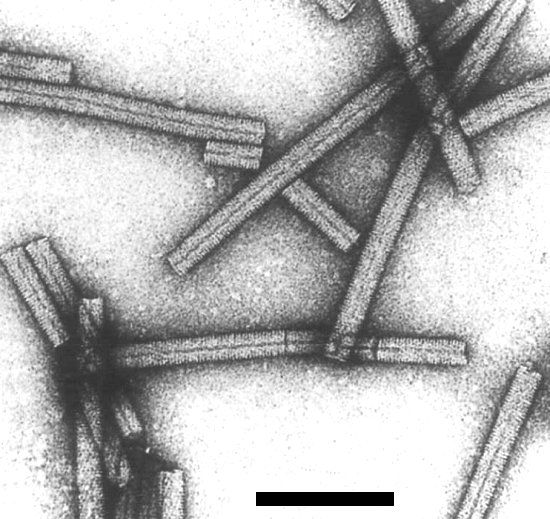
Negatively stained preparation of TMV virions. Bar is 100 nm. Photo courtesy of Oscar Bradfute, Ohio State University.

End view of reassembled TMV coat protein, rotated to reinforce the radial symmetry of 16 1/3 structural units per turn, or 49 protein molecules per turn of the major helix (Markham et al., 1963).
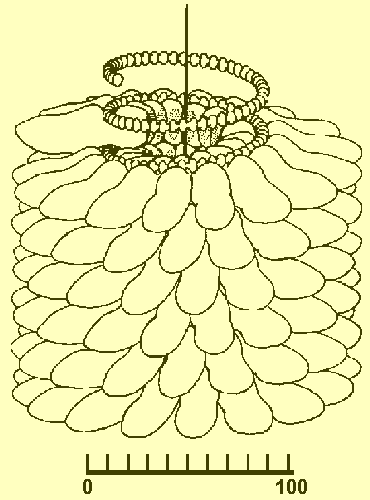
Model of a portion of a virion, showing helically-arrayed protein subunits. Subunits in centre are removed to show the position of the RNA. Scale is in angstroms. From Klug and Caspar (1960), based on the work of R.E. Franklin.

Genetic map of TMV showing the genomic and subgenomic RNAs (thin lines) and the protein products as hatched boxes. Adapted from Sulzinski et al. (1985). Details discussed in Palukaitis and Zaitlin (1986). The 54-kDa protein has not been observed in plants. Drawing provided by James Haudenshield.

Complexes of partially-stripped virions with their associated ribosomes, termed "striposomes" (Wilson, 1984). Photograph courtesy of T.M.A. Wilson. Bar is 300 nm.

Hexagonal TMV virus crystal (left), and cell nucleus (right) in leaf-hair cell of Nicotiana tabacum.
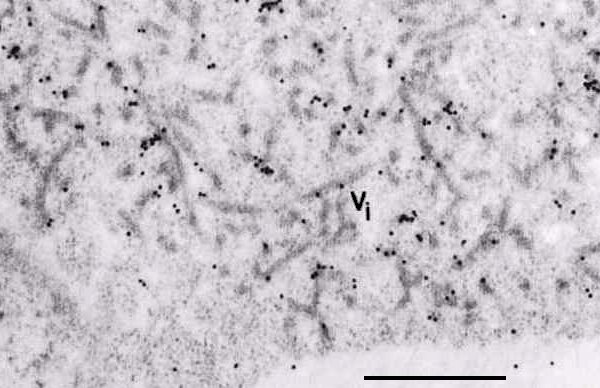
Association of the 126-kDa protein with an X-body. Tissue probed with anti-126-kDa antiserum and immunogold labeled. Vi indicates the viroplasm, or X-body. Bar is 500 nm. From Hills et al. (1987).

Association of TMV movement protein with microtubules in protoplasts of a Nicotiana tabacum cell line. Protoplasts were infected with a mutant of TMV that encodes a fusion protein of the movement protein with a green fluorescent protein. Photograph taken about 18 hr post inoculation, provided by M. Heinlein and R.N. Beachy.
References list for DPV: Tobacco mosaic virus (370)
- Asselin & Grenier, Canadian Journal of Plant Pathology 7: 223, 1985.
- Asselin & Zaitlin, Virology 91: 173, 1978.
- Atkins, Hull, Wells, Roberts, Moore & Beachy, Journal of General Virology 72: 209, 1991.
- Beachy & Zaitlin, Virology 81: 160, 1977.
- Beier, Barciszewska, Krupp, Mitnacht & Gross, EMBO Journal 2: 351, 1984.
- Beijerinck, Verhandelingen der Koninklyke academie van Wettenschappen te Amsterdam 65: 3, 1898. Translated into English as Phytopathological Classics No. 7. American Phytopathological Society Press, St. Paul, MN., 1942.
- Bennett, Phytopathology 29: 1, 1939.
- Brakke, Methods in Virology Vol. 2: eds., Maramorosch & Koprowski, Academic Press, New York, 1967.
- Briand, Moudallal & Van Regenmortel, Journal of Virological Methods 5: 293, 1982.
- Broadbent, Annals of Applied Biology 56: 177, 1965.
- Broadbent & Fletcher, Annals of Applied Biology 52: 233, 1963.
- Bruening, Beachy, Scalla & Zaitlin, Virology 71: 498, 1976.
- Caspar, Advances in Protein Chemistry 18: 37, 1963.
- Cheo & Gerard, Phytopathology 61: 1010, 1971.
- Cooper, Lapidot, Heick, Dodds & Beachy, Virology 206: 307, 1995.
- Dawson, Beck, Knorr & Grantham, Proceedings of the National Academy of Sciences, USA 83: 1832, 1986.
- Ding, Shintaku, Carter & Nelson, Proceedings of the National Academy of Sciences, USA 93: 1115, 1996.
- Dunigan & Zaitlin, Journal of Biological Chemistry 265: 7779, 1990.
- Dunigan, Dietzgen, Schoelz & Zaitlin, Virology 165: 310, 1988.
- Durham & Henry, Virology 77: 510, 1977.
- Esau, Viruses in Plant Hosts, Univ. of Wisconsin Press, Madison, 1968.
- Fraenkel-Conrat & Narita, in Symposium on Protein Structure ed. A.Neuberger, Methuen, London, p. 249, 1958.
- Fraenkel-Conrat & Williams, Proceedings of the National Academy of Sciences, USA 41: 690, 1955.
- Ginoza, Atkinson & Wildman, Science 119: 269, 1954.
- Goelet, Lomonossoff, Butler, Akam, Gait & Karn, Proceedings of the National Academy of Sciences, USA 79: 5818, 1982.
- Golemboski, Lomonossoff & Zaitlin, Proceedings of the National Academy of Sciences, USA 87: 6311, 1990.
- Gooding, in Compendium of Tobacco Diseases eds.H.D. Shew & G.B. Lucas. APS Press, St. Paul, MN, p.44, 1991.
- Hariharasubramanian, Zaitlin & Siegel, Virology 40: 579, 1970.
- Harrington & Schachman, Archives of Biochemistry and Biophysics 65: 278, 1956.
- Harris & Bradley, Virology 52: 295, 1973.
- Harrison & Wilson, Philosophical Transactions of the Royal Society of London, Series B 254: 517, 1999.
- Heinlein, Epel, Padgett & Beachy, Science 270: 1983, 1995.
- Hills, Plaskitt, Young, Dunigan, Watts, Wilson & Zaitlin, Virology 161: 488, 1987.
- Holmes, Phytopathology 24: 845, 1934.
- Holmes, Handbook of Phytopathogenic Viruses. Burgess Publishing Company, Minneapolis, MN. 1939.
- Holmes, Phytopathology 36: 643, 1946.
- Holt, Hodgson, Coker, Beachy & Nelson, Molecular Plant-Microbe Interactions 3: 417, 1990.
- Hosford, Botanical Review 33: 387, 1967.
- Hunter, Hunt, Knowland & Zimmern, Nature 260: 759, 1976.
- Jonard, Richards, Guilley & Hirth, Cell 11: 483, 1977.
- Klug & Caspar, Advances in Virus Research 7: 225, 1960.
- Koh, Song, Lee, Park & Park, Nucleic Acids Research 20: 5474, 1992.
- Kramer & Wittmann, Zeitschrift für Naturforschung B 13: 30, 1958.
- Lauffer, Journal of the American Chemical Society 66: 1188, 1944.
- Lojek & Orlob Science 164: 1407, 1969.
- Mandeles & Bruening, Biochemical Preparations 12: 111, 1968.
- Markham, Frey & Hills, Virology 20: 88, 1963.
- Mayer, Landwirtschaftliche Versuchs-Stationen 32: 451, 1886. Translated into English as Phytopathological Classics No. 7, American Phytopathological Society Press, St. Paul, MN., 1942.
- Meshi, Ishikawa, Takamatsu, Ohno & Okada, FEBS Letters 162: 282, 1983.
- Namba, Pattanayek & Stubbs, Journal of Molecular Biology 208: 307, 1989.
- Nozu & Okada, Journal of Molecular Biology 35: 643, 1968.
- Oberg & Philipson, Biochemical and Biophysical Research Communications 48: 929, 1972.
- Osman & Buck, Journal of Virology 70: 6227, 1996.
- Osman & Buck, Journal of Virology 71: 6075, 1997.
- Padgett, Epel, Heinlein, Watanabe & Beachy, Plant Journal 10: 1079, 1996.
- Palukaitis & Zaitlin, in The Plant Viruses, Volume 2: The Rod-Shaped Plant Viruses. p. 105. ed. M. Van Regenmortel & H. Fraenkel-Conrat, New York, Plenum Press, 1986.
- Paul, Archiv fur Mikrobiologie 30: 304, 1958.
- Powell Abel, Nelson, De, Hoffmann, Rogers, Fraley & Beachy, Science 232: 738, 1986.
- Reinero & Beachy, Plant Molecular Biology 6: 111, 1986.
- Saito, Yamanaka & Okada, Virology 176: 329, 1990.
- Samuel, Annals of Applied Biology 21: 90, 1934.
- Scholthof, Shaw & Zaitlin, Tobacco Mosaic Virus: One Hundred Years of Contributions to Virology. American Phytopathological Society Press, St. Paul, MN. 1999.
- Schramm & Bergold, Zeitschrift für Naturforschung B 2: 108, 1947.
- Shalla, Petersen & Guichedi, Virology 66: 94, 1975.
- Shew & Lucas, Compendium of Tobacco Diseases. American Phytopathological Society Press, St. Paul, MN. 1991.
- Siegel, Virology 46: 50, 1971.
- Siegel & Hudson, Biochimica et Biophysica Acta 34: 254, 1959.
- Siegel & Wildman, Phytopathology 44: 277, 1954.
- Siegel, Zaitlin & Sehgal, Proceedings of the National Academy of Sciences, USA 46: 1845, 1962.
- Silber & Burk, Nature 206: 740, 1965.
- Simón-Buela & García-Arenal, Molecular Plant-Microbe Interactions 12: 112, 1999.
- Stanley, Science 81: 644, 1935.
- Steere, Science 140: 1089, 1963.
- Sulzinski & Zaitlin, Virology 121: 12, 1982.
- Sulzinski, Gabard, Palukaitis & Zaitlin, Virology 145: 132, 1985.
- Valverde, Heick & Dodds, Phytopathology 81: 99, 1991.
- Van Belkum, Abrahams, Pleij & Bosch, Nucleic Acids Research 13: 7673, 1985.
- Van Regenmortel, in The Plant Viruses, Volume 2: The Rod-Shaped Plant Viruses, p. 79, ed. M. Van Regenmortel & H. Fraenkel-Conrat, New York, Plenum Press, 1986.
- Watanabe, Honda, Iwata, Ueda, Hibi & Ishihama, Journal of Virology 73: 2633, 1999.
- Wilson, Virology 137: 255, 1984.
- Wittmann-Liebold & Wittmann, Molecular and General Genetics 100: 358, 1967.
- Wolf, Deom, Beachy & Lucas, Science 246: 377, 1989.
- Zelcer, Weaber, Balazs & Zaitlin, Virology 113: 417, 1981.
- Zimmern, Nucleic Acids Research 2: 1189, 1975.
- Zimmern, Cell 11: 455, 1977.