Details of DPV and References
DPV NO: 381 December 2001
Family: Bromoviridae
Genus: Ilarvirus
Species: Tobacco streak virus | Acronym: TSV
This description also supercedes that for black raspberry latent virus (DPV 106), now regarded as a strain of TSV.
This is a revised version of DPV 307
Tobacco streak virus
S. W. Scott Department of Plant Pathology and Physiology, Clemson University, Clemson, SC 29634-0377, USA.
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Johnson (1936)
- Synonyms
- Annulus orae (Thomas & Zaumeyer,
1950)
- Asparagus stunt virus (Brunt & Paludan, 1970)
- Black raspberry latent virus (Jones & Mayo, 1975)
- Datura 'quercina' virus (Edwardson & Purciful, 1974)
- Nicotiana virus 8 (Smith, 1937)
Strawberry necrotic shock virus (Stace-Smith & Frazier, 1971) - Asparagus stunt virus (Brunt & Paludan, 1970)
A virus with quasi-isometric particles 27 - 35 nm in diameter. It has a wide host range and occurs worldwide but not often in epidemic proportions. Virus particle preparations contain at least four ssRNA species of 3.49, 2.96, 2.2 and approx 0.9 kb and a major protein of Mr 28,000. It is unstable in plant extracts but can be transmitted mechanically to a number of hosts. It is transmitted through pollen, seed, and by thrips.
Main Diseases
Causes systemic necrotic lines bordering veins (Fig. 1) (streak, `necrose branca') in tobacco, with subsequent recovery in newly developing leaves; mottling or no symptoms in dahlia (Costa & Carvalho, 1961; Brunt, 1968); mottling in cotton (Costa & Carvalho, 1961), Trifolium pratense and Melilotus alba; yellow ringspots and malformation in tomato (Martelli & Cirulli, 1969; Costa et al., 1961); stunting of asparagus (Brunt & Paludan, 1970); vein yellowing in rose (Fulton, 1970b); red node in bean (Thomas & Zaumeyer, 1950); systemic necrosis in pea (Patino & Zaumeyer, 1959), soybean (Costa & Carvalho, 1961; Fagbenle & Ford, 1970) and potato (Costa et al., 1964); mild mosaic in potato (Salazar et al., 1982); and necrotic shock in strawberry (Stace-Smith & Frazier, 1971) (Fig. 2). It has also been isolated from black raspberry (Converse, 1972), red raspberry (Stace-Smith et al., 1982), blackberry (Jones & Mayo, 1975), groundnut, pepper (Gracia & Feldman, 1974), globe artichoke (Costa & Tasaka, 1971), alfalfa (Paliwal, 1982), sunflower (Dijkstra, 1983; Ravi et al., 2001), lettuce and escarole (McDaniel et al., 1992) and numerous wild species including Ageratum houstonianum in Australia (Greber et al., 1991); Rumex spp, Taraxacum officinale (Fulton, 1948), Brassica campestris, Silybum marianum, and Rhaphanus rhaphinastrum in the USA (Cupertino et al., 1984); and Talinum patens in Brazil (Lima Neto, 1984). The virus has been identified as infecting ornamental species including: Alstroemeria (Brunt & Phillips, 1981), Clematis (Bellardi et al., 1985; Rana et al., 1987), Lisianthus (Freitas et al., 1996), Impatiens (Lockhart & Betzold, 1980) and Ajuga reptans (Shukla & Gough, 1983).
Geographical Distribution
Probably occurs worldwide. Reported from Europe, North and South America, South Africa, India, Japan, Australia, and New Zealand.
Host Range and Symptomatology
The host range is wide; many species in over 30 monocotyledonous and dicotyledonous families are susceptible. Extensive lists of susceptible species have been prepared by Edwardson & Christie (1991) and Brunt et al. (1996).
- Diagnostic species
Nicotiana tabacum (tobacco). Local necrotic spots or rings (Fig. 3), systemic necrotic lines and 'oak leaf' patterns; plants recover from necrotic symptoms and leaves that expand several weeks after infection may appear healthy. With many strains of the virus, these apparently healthy leaves have dentate rather than entire margins (Berkeley & Phillips, 1943) and petal terminations are filamentous (Costa, 1945). Isolates from Rubus and dahlia infect tobacco with difficulty or not at all (Fulton, 1985) although successful transmission of isolates from Rubus to herbaceous hosts, including tobacco, has been reported when using 2% nicotine (Jones & Mayo, 1975), 2% nicotine plus aluminium oxide powder (Converse & Lister, 1969), or 1% nicotine plus 1% polyvinylpyrrolidone (Brunt & Stace-Smith, 1976).
Cyamopsis tetragonolobus (guar). Small, dark local lesions.
Vigna unguiculata ssp. cylindrica (catjang). Strains may give either local reddish necrotic lesions or chlorotic lesions with subsequent systemic necrosis or mottle.
- Propagation species
Cultures of most isolates are readily maintained in tobacco or Vinca rosea. Tobacco, Nicotiana rustica, Datura stramonium and V. unguiculata ssp. cylindrica are good sources of virus for purification. Yields of many strains are several times higher when propagated in D. stramonium than when propagated in other species (Fulton & Potter, 1971). Isolates from Rubus are best propagated in Chenopodium quinoa (Fig. 4 and Fig. 5).
- Assay species
V. unguiculata ssp. cylindrica, Dolichos biflorus, Beta patellaris, Phaseolus vulgaris cv. Black Turtle (Fig. 6) and Gomphrena globosa have been used as local lesion hosts with various isolates.
Strains
Many variants exist. Serological variants are described from South America and North America (Fulton, 1972; Ghabrial & Lister, 1974; Gooding, 1971; Kaiser et al., 1982) and from Rubus (Jones & Mayo, 1975). Several isolates differ in symptoms caused on indicator hosts. Isolates from Rubus (Converse, 1972; Jones & Mayo, 1975) and dahlia (Fulton, 1985) are transmitted to tobacco only with difficulty or not at all. Black raspberry latent virus (Converse & Lister, 1969; Jones & Mayo, 1975) and Bean red node virus (Thomas & Zaumeyer, 1950) are considered distinct strains. Isolate SB10 from potato causes markedly different symptoms from those described for another isolate of TSV from potato (Costa et al., 1964), suggesting that the two isolates are distinct strains (Salazar et al., 1982). Nucleic acid hybridization and ELISA showed an isolate of strawberry necrosis virus and a TSV isolate from Rubus to be closely related but distinct from another group of isolates (the WC isolate from white clover, Bean red node virus and an isolate from tobacco) (Stenger et al., 1987). An isolate of TSV from peanut in S. Africa showed greater than 95% homology with the nucleic acid sequence of the WC isolate (Scott et al., 1998).
Transmission by Vectors
A Frankliniella sp. is reported as a vector in Brazil (Costa & Lima Neto, 1976). A mixture of Thrips tabaci and F. occidentalis was reported to transmit the virus in USA (Kaiser et al., 1982). T. tabaci transmitted the virus to C. quinoa (Sdoodee & Teakle, 1987) apparently by mechanical inoculation rather than involving ingestion of the virus by the vector (Sdoodee & Teakle, 1993). A combination of thrips (Microcephalothrips abdominalis) and wind-blown pollen is reported to spread the virus from Ageratum houstonianum to adjacent crops of tobacco (Greber et al., 1991).
Transmission through Seed
Reported for bean, D. stramonium, C. quinoa (Brunt, 1969), M. alba, Glycine max, Gomphrena globosa, Nicotiana clevelandii, V. unguiculata (Kaiser et al., 1982), black raspberry (Converse & Lister, 1969), tomato (Sdoodee & Teakle, 1988), and Nicandra physalodes (Salazar et al., 1982). Transmitted through seed of strawberry hybrids via pollen or ovule. Frequencies of transmission range from 90% in G. max, 35% in strawberry (Johnson et al., 1984), to less than 1% in V. unguiculata (Kaiser et al., 1982). The Black raspberry latent strain is reported to be transmitted by pollen to the plant pollinated (Converse & Lister, 1969).
Transmission by Dodder
Transmitted by Cuscuta campestris (Fulton, 1948).
Serology
The virus is poorly immunogenic. Intravenous injection of substantial amounts of virus at short intervals yielded antisera of low titre (Gooding, 1971) as did four intramuscular injections of 3 mg of virus at 5-7 day intervals followed by 2 intravenous injections (Ghanekar & Schwenk, 1980). Two intramuscular injections mixed 1:1 with Freund's incomplete adjuvant followed by 4 intravenous injections yielded antisera with a titre of 1:32 (Converse, 1972). Fixation of the virus with 0.2% formaldehyde prior to immunization and a series of 6 intravenous immunizations at 3-5 day intervals did not produce antisera of high titre (Jones & Mayo, 1975). However, all the sera produced by the above methods functioned in gel double diffusion serological assays. High titre sera (1:2,560-1:5,120 in microprecipitin tests) have been reported on one occasion when an extended schedule of 12-14 intramuscular injections of 1-2 mg of virus in Freund's incomplete adjuvant at 3-4 day intervals was used (Fulton, 1985). The virus gives a well-defined zone of precipitate in agar gels containing saline. There is serological variation among isolates of TSV with immunodiffusion assays frequently revealing spurs indicating antigenic differences. Strain-specific antisera have been prepared by cross absorption with purified particles of various virus strains. Monoclonal antibodies have been produced that recognized 4 antigenic differences and were able to distinguish between isolates of TSV from soybean, grape, rose, and tobacco (Halk et al., 1984).
Nucleic Acid Hybridization
cDNA probes have been used for the detection of strawberry necrotic shock virus in strawberry (Stenger et al., 1987).
Relationships
Some isolates cross-react with each other's antisera, but some have strain-specific antigenic determinants. Many strains reciprocally protect against each other in plants, but pairs of strains that protect only unilaterally, or not at all, are reported (Fulton, 1978). The inability to cross-protect is not necessarily correlated with serological difference (Fulton, 1985). TSV is the type member of the genus Ilarvirus, one of 5 genera contained within the family Bromoviridae. Species in the genus Ilarvirus were placed into 7 subgroups based largely on serological relationships (Roossinck et al., 2000). Viruses within a subgroup are serologically related but show no serological relationship to members of other subgroups. In recent years nucleotide sequence data has been used to confirm or alter the relationships among the subgroups. TSV was for many years the sole member of subgroup 1. More recently, sequence data now indicate that Parietaria mottle virus (PMoV) (originally the sole member of subgroup 6) should be included in subgroup 1 (S.W. Scott, unpublished data). Lisianthus line pattern virus (LLPV), that has serological relationships with both TSV and PMoV (Lisa et al., 1994), should also be included in this subgroup. Hydrangea mosaic virus (HdMV) was reported to be a member of subgroup 1 based on sequence data (Ge & Scott, 1996). However, it has since been determined that the virus isolate sequenced was PMoV and not HdMV and that HdMV is closely related to Elm mottle virus (EMoV) in subgroup 2 (S.W. Scott, unpublished data).
Stability in Sap
Undiluted tobacco sap loses most of its infectivity within 5 min after extraction (Fulton, 1949) and all of its infectivity in less than 36 h. Infectivity is lost more slowly in diluted extracts and is stabilized in extracts by the presence of antioxidants, especially 2-mercaptoethanol. The thermal inactivation point for most isolates is between 53 and 64°C and the dilution endpoint between 1/30 and 1/15,625. Variation in these properties depends on the host species from which the sap was prepared and on the presence or absence of antioxidants (Brunt et al., 1996). The virus remains infective for many years in diced tissue dried and stored at about 2°C and in freeze-dried tissue stored at -20°C.
Purification
The following methods are effective (Fulton, 1967). Harvest infected leaf tissue about 10 days after inoculation and homogenize in 0.02 M phosphate, pH 8.0, containing 0.02 M 2-mercaptoethanol and Al2O3 (1g leaf: 1.4 ml buffer: 0.15g Al2O3). After centrifuging for 15-20 min at 1,500 g, thoroughly mix the supernatant fluid with hydrated calcium phosphate (0.8ml : 1g tissue). Centrifuge at 1,500 g for 15-20 min and centrifuge the supernatant fluid for 3 h at 78,000 g. Resuspend the pellets in 0.01 M EDTA, pH 6.0, adjust the pH to 5.0 with citric acid and remove the precipitate by centrifugation. Readjust the supernatant fluid containing the virus, to pH 6.0 with NaOH and concentrate the virus by high speed centrifugation. Resuspend the pellets in 0.01 M EDTA, pH 6.0, in which the virus is more stable than in either water or several different buffers (Fulton, 1970a). Yields of virus from infected D. stramonium may be up to 400 µg per gram of tissue (Fulton & Potter, 1971). Some virus isolates cannot be purified in this way and require a method involving precipitation with 4% polyethylene glycol (mol. wt 6000) and 0.1 M NaCl (Fulton, 1978).
The method described by van Vloten-Doting (1975) for purification from N. edwardsonii using Triton X-100 is useful for the WC isolate (S.W. Scott, unpublished data) and procedures involving clarification of leaf extracts by charcoal and freeze/thawing (Mink et al., 1966) and di-ethyl ether-CCl4 (Greber, 1971) have also been useful.
Properties of Particles
The virus has three main kinds of nucleoprotein particle (Fig. 7), designated T, M and B, with sedimentation coefficients (s20,w) of c. 90, 98, and 113 S, respectively (Lister & Bancroft, 1970). The relative proportions of the particle types may vary with the host, purification procedure (Lister & Bancroft, 1970), virus strain, or time after inoculation (Fulton & Potter, 1971). Infectivity of separated particle types is nil or nearly so for T and M particles, and low for B particles (Fulton, 1970a). Maximum infectivity is obtained with mixtures of M and B particles and increases further with the addition of T particles. However, increasing the proportion of T particles in mixtures of M+B of constant concentration decreases infectivity (Fulton, 1975).
Mol. wt (x 10-6) of T, M and B particles are 4.72, 5.92 and 7.45 respectively (Ghabrial & Lister, 1974).
Diffusion coefficients (x 10-7 cm2.sec-1) of T, M and B particles are 1.39, 1.32 and 1.23 respectively.
The isoelectric point of two strains was pH 4.6 in 0.1 ionic strength buffer.
Partial specific volume: 0.71 ml/g.
A260 nm (1 µg/ml, 1 cm light path): 5.1.
Buoyant density in CsCl: 1.35 g.cm-3 for each particle type (Jones & Mayo, 1975).
Particle Structure
Particles are quasi-isometric and of three major sizes: 27, 30, and 35 nm in diameter (Lister et al., 1972). The numbers of protein subunits for the three particle types are thought to be: T, 142; M, 179; and B, 225 (Ghabrial & Lister, 1974). Regularity in arrangement of subunits is not apparent in electron micrographs, which show slightly distorted particles without hollow centres (Fig. 7).
Particle Composition
Nucleic acid: Virus particles contain ssRNA of which 3 are genomic and up to 2 others are subgenomic. Generally, the genomic RNA-3 and the subgenomic RNA-4 are present in much greater concentration than the other species. Virus particle preparations have A260/A280 of c. 1.56. Particles contain c. 14% RNA (Ghabrial & Lister, 1974). The complete nucleotide sequences of the 3 genomic species of the WC isolate have been determined; they contain 3,491 nt (RNA-1; accession no. U80934; Scott et al., 1998), 2,926 nt (RNA-2; accession no. U75538, Scott et al., 1998) 2,205 nt (RNA-3; accession no. X00435 (Cornelissen et al., 1984). The subgenomic RNA-4 is a copy of the coat protein gene located at the 3' end of RNA-3. The exact size is unknown. A subgenomic RNA-4a occurs in preparations of the WC isolate and, based on comparisons with work on Elm mottle virus, is thought to be a copy of the 2b ORF. The exact size of this molecule is unknown (Scott et al., 1998).
Protein: Comprises 86% of the particle weight. The Mr of the protein subunits is 28,000-30,000 estimated by electrophoresis in PAGE. The amino acid composition of two serologically different strains differed, especially in contents of alanine, aspartic acid, leucine, proline and valine (Ghabrial & Lister, 1974). The putative translation product of the CP gene of accession no. X00435 contains 237 aa and has an Mr of 26,240. The coat protein contains a conserved arginine motif that is responsible for the binding of the protein in the activation of the genome of TSV, other ilarviruses, and Alfalfa mosaic virus (AMV) (Ansel-McKinney et al., 1996).
Genome Properties
The genome comprises RNA-1, RNA-2 and RNA-3 (Fig. 8). RNA-1 contains a single ORF that encodes a putative polypeptide (1a protein) of 123 kDa. The polypeptide contains two domains with sequence characteristics of methyltransferase and NTP-binding activities. RNA-2 contains 2 ORFs. The putative product of the larger ORF (2a protein) is 91.5 kDa and contains a domain with sequence characteristics of an RNA polymerase. The smaller 2b ORF begins within the larger ORF and extends towards the 3' terminus of the molecule. The putative product (2b protein) is 22 kDa and appears to be expressed via a subgenomic RNA-4a. (Scott et al., 1998). RNA-3 is bicistronic and codes for a putative movement (3a) protein of 31.6 kDa and the coat (3b) protein of 26.2 kDa. The coat protein gene is expressed via subgenomic RNA-4. Mixtures of the three main RNA species are not infective, but become so in the presence of either subgenomic RNA-4 or the coat protein. Coat protein of AMV, although unrelated serologically to that of TSV, also activates infectivity. Similarly, the coat protein of TSV activates the three largest RNA molecules of AMV (van Vloten-Doting, 1975). TSV coat protein also activated nucleic acid mixtures of Citrus leaf rugose virus and of Citrus variegation virus, ilarviruses that are unrelated serologically to TSV (Gonsalves & Garnsey, 1975). By inference from the work on AMV (Houser-Scott et al., 1994; Reusken, et al.,1994) the AUGC motifs in the 3' UTR of the virus are involved in the activation of infectivity.
van Vloten-Doting (1975) reported that the largest genomic RNA molecule occurs in B particles, the intermediate RNA molecule in M particles, and the smallest RNA molecule in T particles. However, heterogeneity in the RNA content of M particles has been described (Clark & Lister, 1971), and this agrees with genetic data indicating that determinants for lesion type in V. unguiculata ssp. cylindrica may be located in either T or M particles (Fulton, 1970a). A severe dwarfing symptom in TSV-infected Turkish tobacco is determined by two genes, one contained on RNA in M particles and the other on RNA in B particles (Fulton, 1972). The size and colour of lesions in Vigna are inherited independently, the gene for small lesions being frequently carried on RNA-3 in T particles (Fulton, 1975). An unstable gene conditioning a `non-recovery' symptom in tobacco was carried on only RNA-3 in T particles (Fulton, 1985). The presence of many minor species of RNA was noted in a mild, infrequently transmitted isolate of TSV. Absence of the minor RNAs was correlated with the development of atypically severe symptoms on C. quinoa and P. vulgaris (Walter et al., 1995).
Relations with Cells and Tissues
Virus particles occur in aggregates in the cytoplasm and nucleus of hosts (Edwardson & Purcifull, 1974). In soybean, the virus was obtained only from the embryos of mature seeds but was present in both the embryos and coats of immature seeds (Ghanekar & Schwenk, 1980). In cotton the virus does not become systemic unless 'anthocyanosis virus' is also present (Costa, 1969).
Notes
Symptoms in diagnostic hosts may vary and may be confused with those of other viruses. Identification should be made by serology and PCR. In some instances single isolates of the virus have been described as strains without evidence that they differ significantly from other isolates. Some of the data in this description were obtained with only one or two isolates and may not be representative of all isolates. Lisianthus line pattern virus (Lisa et al., 1994), although reported as a new ilarvirus, has not been included in the current ICTV list of ilarviruses. TSV has recently been reported in American cranberry (Vaccinium macrocarpon) (Jones et al., 2001) and has been identified as the recent cause of major crop loss in sunflower and groundnut in India (Ravy et al., 2001; Reddy et al., 2001).
Acknowledgements
Figures by R.H. Converse and R. Stace-Smith reprinted from Virus Diseases of Small Fruits, Agriculture Handbook 631, 1987 with permission of USDA/ARS and supplied by Dr. R.R. Martin, USDA-ARS 3420 NW Orchard Ave, Corvallis, OR 97330.
Figures

Systemic necrotic lines bordering veins in Nicotiana clevelandii 12 days after inoculation with a TSV isolate from Rubus.

Necrosis of young leaves in Fragaria vesca var. semperflorens cv. Alpine 2wk after leaf grafting from a strawberry plant infected with TSV.

Chlorotic/necrotic local lesions in Nicotiana tabacum cv. `Haranova', 5 days after inoculation with an isolate of TSV from Rubus.

Chlorotic local lesions in Chenopodium quinoa 6 days after inoculation with an isolate of TSV from Rubus.

Systemic tip necrosis of Chenopodium quinoa 21 days after inoculation with an isolate of TSV from Rubus.
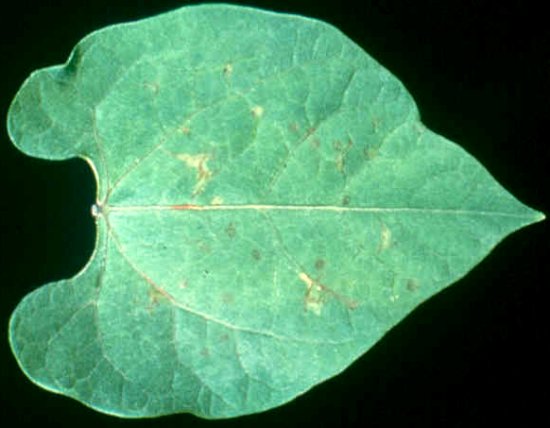
Necrotic local lesions in a leaf of Phaseolus vulgaris cv. `Black Turtle', 5 days after inoculation with an isolate of TSV from Rubus.

Electron micrograph of a purified particle preparation of an isolate of TSV from Rubus fixed in glutaraldehyde and negatively stained. The bar represents 100 nm.
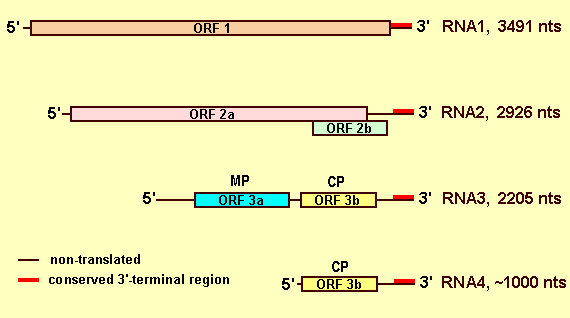
Genome diagram of the virus. MP, movement protein; CP, coat protein. RNAs 1-3 are genomic and act as mRNAs. The ORFs 2b and 3b (CP) are expressed from subgenomic RNAs. It is not known whether the subgenomic form from ORF2b is encapsidated. The subgenomic RNA4 is encapsidated and the presence of either it or the coat protein is required for infectivity.
References list for DPV: Tobacco streak virus (381)
- Ansel-McKinney, Scott, Swanson, Xin & Gehrke, The EMBO Journal 15: 5077, 1996.
- Bellardi, Credi & Gelli, Phytopathologia Mediterranea 24: 255, 1985.
- Berkeley & Phillips, Canadian Journal of Research, Section C 21: 181, 1943.
- Brunt, Plant Pathology 17: 119, 1968.
- Brunt, Report of the Glasshouse Crops Research Institute for 1968: 104, 1969.
- Brunt & Paludan, Phytopathologische Zeitschrift 69: 277, 1970.
- Brunt & Phillips, Report of the Glasshouse Crops Research Institute for 1979: 151, 1981.
- Brunt & Stace#Smith, Acta Horticulturae 66: 71, 1976.
- Brunt, Crabtree, Dallwitz, Gibbs, Watson & Zurcher, (eds.)`Plant Viruses Online: Descriptions and Lists from the VIDE Database. Version: 9th April 1997 .' URL http://biology.anu.edu.au/Groups/MES/vide/, 1996.
- Clark & Lister, Virology 45: 61, 1971.
- Converse, Phytopathology 62: 1001, 1972.
- Converse & Lister, Phytopathology 59: 325, 1969.
- Cornelissen, Janssen, Zuidema & Bol, Nucleic Acids Research 12: 2427, 1984.
- Costa, Phytopathology 35: 1029, 1945.
- Costa, Phytopathologische Zeitschrift 65: 219, 1969.
- Costa & Carvalho, Phytopathologische Zeitschrift 43: 113, 1961.
- Costa & Lima Neto, Fitopatologi 11: 11, 1976.
- Costa & Tasaka, Biologico 37: 176, 1971.
- Costa, Arvalho, Oliveira & Deslandes, Bragantia 20: cvii, 1961.
- Costa, Carvalho & Deslandes, Bragantia 23: 1, 1964.
- Cupertino, Grogan, Petersen & Kimble, Plant Disease 68: 331, 1984.
- Dijkstra, Netherland Journal of Plant Pathology 89: 153, 1983.
- Edwardson & Christie, Handbook of Viruses Infecting Legumes, CRC press, 504 pp, 1991.
- Edwardson & Purcifull, Phytopathology 64: 1322, 1974.
- Fagbenle & Ford, Phytopathology 60: 814, 1970.
- Freitas, Kitajima & Rezende, Plant Disease 80: 1080, 1996.
- Fulton, Phytopathology 38: 421, 1948.
- Fulton, Phytopathology 39: 231, 1949.
- Fulton, Virology 32: 153, 1967.
- Fulton, Virology 41: 288, 1970a.
- Fulton, Plant Disease Reporter 54: 949, 1970b.
- Fulton, Virology 50: 810, 1972.
- Fulton, Virology 67: 188, 1975.
- Fulton, Virology 85: 1, 1978.
- Fulton, A.A.B. Descriptions of Plant Viruses 307, 1985.
- Fulton & Potter, Virology 45: 734, 1971.
- Ge & Scott, Virus Research 40: 57, 1996.
- Ghabrial & Lister, Virology 57: 1, 1974.
- Ghanekar & Schwenk, Phytopathologische Zeitschrift 97: 148, 1980.
- Gonsalves & Garnsey, Virology 67: 319, 1975.
- Gooding, Phytopathology 61: 1303, 1971.
- Gracia & Feldman, Phytopathologische Zeitschrift 80: 313, 1974.
- Greber, Queensland Journal of Agricultural and Animal Sciences 28: 105, 1971.
- Greber, Klose, Teakle & Milne, Plant Disease 75: 450, 1991.
- Halk, Hsu, Aebig & Franke, Phytopathology 74: 367, 1984.
- Houser-Scott, Baer, Liem, Cai & Gehrke, Journal of Virology 68: 2194,1994.
- Johnson, Phytopathology 26: 285, 1936.
- Johnson, Converse, Amorao, Espejo & Frazier, Plant Disease 68: 390, 1984.
- Jones & Mayo, Annals of Applied Biology 79: 297, 1975.
- Jones, McGavin & Dolan, Online. Plant Health Progress, http://www.planthealthprogress.org/current/research/tsv/, 2001.
- Kaiser, Wyatt & Pesho, Phytopathology 72: 1508, 1982.
- Lima Neto, Fitopatologia Brasileira 9: 637, 1984.
- Lisa, Vaira, d'Aquilio, Dellavalle, Masenga, Milne & Boccardo, Acta Horticulturae 377: 81 1994.
- Lister & Bancroft, Phytopathology 60: 689, 1970.
- Lister, Ghabrial & Saksena, Virology 49: 290, 1972.
- Lockhart, & Betzold, Plant Disease 64: 289, 1980.
- McDaniel, Raid, Elliott, Tsai, & Nagata, Plant Disease 75: 966 1992.
- Martelli & Cirulli, Phytopathologia Mediterranea 8: 154, 1969.
- Mink, Saksena & Silbernagel, Phytopathology 56: 645, 1966.
- Paliwal, Canadian Journal of Plant Pathology 4: 175, 1982.
- Patino & Zaumeyer, Phytopathology 49: 43, 1959.
- Rana, Krajacic, Stefanac, Plese, Rubino & Milicic, Annals of Applied Biology 111: 153, 1987.
- Ravi, Buttgereitt, Kitkaru, Deshmukh, Lesemann & Winter, Plant Pathology 50: 800, 2001.
- Reddy, Prasada Rao, Thirumala-Devi, Reddy, Mayo, Roberts, Satyanarayana, Subramaniam & Reddy, Plant Disease 86: (in press) 2002.
- Reusken, Neeleman & Bol, Nucleic Acids Research 22: 1346, 1994.
- Roossinck, Bujarski, Ding, Hajimorad, Hanada, Scott & Tousignant, in Virus Taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses, eds M. H. V. Regenmortel et al, 1162pp, 2000.
- Salazar, Abad & Hooker, Phytopathology 72: 1550, 1982.
- Scott, Zimmerman & Ge, Archives of Virology, 143: 1187, 1998.
- Sdoodee & Teakle, Plant Pathology 36: 377, 1987.
- Sdoodee & Teakle Australian Journal of Agricultural Research 39: 469, 1988.
- Sdoodee & Teakle, Plant Pathology 42: 88, 1993.
- Shukla & Gough, Plant Disease 67: 221, 1983.
- Smith, A Textbook of Plant Virus Diseases, Churchill Press, 259pp, 1937
- Stace-Smith & Frazier, Phytopathology 61: 757, 1971.
- Stace-Smith, Daubeny, Bristow & Baumann, Acta Horticulturae 129: 91, 1982.
- Stenger, Mullins & Morris, Phytopathology 77: 1330, 1987.
- Thomas & Zaumeyer, Phytopathology 40: 832, 1950.
- van Vloten-Doting, Virology 65: 215, 1975.
- Walter, Wyatt & Kaiser, Phytopathology 85: 1394, 1995.