Details of DPV and References
DPV NO: 385 December 2001
Family: Secoviridae
Genus: Nepovirus
Species: Grapevine fanleaf virus | Acronym: GFLV
This is a revised version of DPV 28
Grapevine fanleaf virus
G. P. Martelli Dipartimento di Protezione delle Piante, Univerità degli Studi and Centro di Studio del CNR sui Virus e le Virosi delle Colture Mediterranee, 70126 Bari, Italy
B. Walter IUP Valorisationa et Transformation des Productions Agricoles, Université de Haute-Alsace, 68008 Colmar, France
L. Pinck Institut de Biologie Moléculaire des Plantes, CNRS, 67084 Strasbourg, France
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Cadman et al. (1960); Dias & Harrison (1963); Quacquarelli et al. (1976).
For disease description see Hewitt (1954); Bovey et al. (1980).
Synonyms
Grapevine roncet virus (Viala, 1893)
Grapevine court-noué virus (Coste-Floret, 1896)
Grapevine Reisigkrankheit virus (Cholin, 1896)
Grapevine arricciamento virus (Pantanelli, 1910)
Grapevine degenerazione infettiva virus (Petri, 1918)
Grapevine urticado virus (Dias, 1950)
A virus with isometric particles with angular outline, about 30 nm in diameter, occurring worldwide in Vitis species. Virus particles contain a single protein species of Mr 56,000. The virus has a linear bipartite positive sense ssRNA genome; the two RNA species are encapsidated in separate particles. The virus is a grapevine pathogen, can be transmitted by inoculation of plant sap and infects a moderate range of experimental herbaceous hosts. It is spread over medium and long distances in infected propagative material and soil, and between plants in the field by dorylamoid nematodes.
Main Diseases
The virus is the agent of fanleaf and yellow mosaic diseases of grapevines (Hewitt et al., 1962; Martelli & Hewitt, 1963a; Vuittenez, 1963; Taylor & Hewitt, 1964). The vein banding disease (Fig. 1) originally thought to be induced by a specific virus strain (Hewitt et al, 1972), results from a mixed infection by grapevine yellow speckle viroid and GFLV (Krake & Woodham, 1983; Szychowski et al., 1995). Fanleaf disease is characterized by malformations of leaves (open marginal and petiolar sinuses, prominent marginal teeth, asymmetrical blades, irregular veins) (Fig. 2) and canes (uneven internode spacing, double nodes, zigzag growth, abnormal branching, fasciations, flattening) (Fig. 3), and by various types of chlorotic discolourations of the foliage (mottling, yellowing, ringspots, line patterns). Bunches are reduced in number and size, ripen irregularly and show shot berries and poor berry setting (Fig. 4). Yellow mosaic is typically characterized by various patterns of brilliant chrome-yellow discolourations of leaves and shoots (Fig. 5, Fig. 6 and Fig. 7). Yellowing is most prominent in spring, fading away as the season progresses (heat masking). Endocelluar cordons (trabeculae), i.e. abnormal ribbon-shaped straight bodies made up primarily of cellulose (Graniti & Russo, 1965) cross the lumen of vascular elements of infected vines (Petri, 1913; Gifford et al., 1956). Crop losses range from moderate (5-10%) to very high (up to 90% or more) according to the virulence of the virus strain and varietal susceptibility. Fruit quality is also affected by a decrease in sugar content and titratable acidity. American rootstocks suffer a decrease of pruning wood up to 50% and show lower rooting ability of cuttings and graft take (Walter & Martelli, 1996). There is apparently no correlation between symptom severity in grapevines and virus titre (Frantz & Walker, 1995).
Geographical Distribution
Occurs in all regions of the world wherever Vitis vinifera and hybrid rootstocks of the grapevine are grown. The virus is apparently native to V. vinifera and originated in the same area as the natural host (Hewitt, 1968).
Host Range and Symptomatology
Vitis spp. is the major natural host, but the virus can occasionally infect weeds (Horvath et al., 1994). The virus is readily transmitted by inoculation of grapevine sap expressed in the presence of 2.5% nicotine in water or in 0.1M phosphate buffer. The experimental host range is moderate, comprising about 35 species in six different families (Brückbauer & Rüdel, 1961a; Baldacci et al., 1962; Hewitt et al., 1962; Dias, 1963; Martelli & Hewitt, 1963a; Vuittenez, 1963; Taylor & Hewitt, 1964).
Diagnostic species
Chenopodium amaranticolor and C. quinoa. Chlorotic/necrotic local lesions develop in 7-10 days. Systemically infected leaves show mottling, vein clearing, and deformation depending on the virus strain (Fig. 9). Infected plants are stunted. Symptoms fade as the plants age.
Gomphrena globosa. Chlorotic local lesions in 7-8 days soon turning reddish, light green to yellow spots and twisting of systemically invaded upper leaves (Fig. 10).
Nicotiana benthamiana and N. clevelandii. Occasional faint yellowish lesions followed by systemic mottling and deformation of the leaves in 10 to 15 days.
The above hosts are infectible with and develop symptoms with most virus strains whereas others, such as Phaseolus vulgaris, Cucumis sativus, and Petunia hybrida, are infected by a smaller number of strains.
Propagations species
Assay species
Strains
Differences in symptomatology in grapevine distinguish two major groups of biological variants i.e. distorting strains, associated with malformation of leaves and canes (Fig. 2, Fig. 3, Fig. 4) and chromogenic strains, associated with chrome yellow discolourations of the foliage (Fig. 5, Fig. 6, Fig. 7) (Hewitt et al., 1962). Serological variants are rare. One such variant from Tunisia was identified by gel double diffusion tests (Savino et al., 1985) and others, from five different countries, were distinguished by monoclonal antibodies (Huss et al., 1987).
Transmission by Vectors
Distorting and chromogenic virus strains are transmitted by the longidorid nematode Xiphinema index (Hewitt et al., 1958, 1962). X. italiae is also an occasional vector (Cohn et al., 1970). All larval stages of X. index transmit but lose this ability after moulting. Adults retain infectivity for several months even when held in the root zone of a virus-immune host (Raski et al., 1965). Virus can be acquired or transmitted within a few minutes of feeding (Das & Raski, 1968). Virus particles are associated specifically with the cuticular lining of the odontophore, where the maximum concentration of particles usually occurs, the slender esophagus, and the esophageal pump (Raski et al., 1973; Taylor & Robertson, 1970). Vectors do not transmit virus to their progeny. Populations of X. index transmit local virus isolates with a higher efficiency than those from other geographical areas (Catalano et al., 1989). Seedlings and rooted cuttings of V. vinifera and V. rupestris are good virus bait plants in studies with nematode vectors.
Transmission through Seed
The virus is abundant in the endosperm of seeds from infected vines (Cory & Hewitt, 1968) and can occasionally be transmitted to seedlings (Lazar et al., 1990). The virus occurs in pollen of infected grapevines and herbaceous hosts (Cory & Hewitt, 1968) and is seed-transmitted in C. amaranticolor (Dias, 1963), C. quinoa (Brückbauer & Rüdel, 1961a) and soybean (Cory & Hewitt, 1968).
Transmission by Grafting
The virus is readily transmitted from vine to vine by grafting, which has been the major mechanism for its dissemination worldwide. The specific virus indicator V. rupestris develops typical symptoms following cleft-, bench-, or green-grafting (Fig. 8) (Martelli, 1993; Lahogue et al., 1995).
Transmission by Dodder
The virus was not transmitted to C. amaranticolor by Cuscuta campestris (Dias 1963).
Serology
The virus is a moderate immunogen with polyclonal antisera giving titres up to 1/1024. The virus forms a single precipitin line in gel double diffusion tests, granular precipitates in microprecipitin tests, and virus particles decorate uniformly in immuno-electron microscopy tests (Dias & Harrison, 1963; Martelli & Hewitt, 1963b; Vuittenez & Kuszala, 1963; Taylor & Hewitt, 1964; Russo et al., 1980). Fifteen mouse monoclonal antibodies to a French virus isolate were produced (Huss et al., 1987). Leaves collected in spring, rootlets, and cortical scrapings from mature canes are good antigen sources for serological assays (Huss et al., 1986; Rowhani et al., 1992; Boscia et al., 1997). Scrapings from mature canes can be used without apparent loss of virus titre after storage in the cold for many months (Walter & Etienne, 1987; Rowhani et al., 1992). Serological assays for the virus in grapevine extracts, once done by the latex agglutination test (Bercks & Querfurth, 1969), are now routinely done by ELISA using either polyclonal (Walter et al., 1984; Rowhani et al., 1992) or monoclonal antibodies (Huss et al., 1986; Huss & Walter, 1987). ELISA was used for detecting the virus in extracts from X. index individuals hand-picked from the field (Catalano et al., 1991; Esmenjaud et al., 1992).
Nucleic Acid Hybridization
Radioactive (Fuchs et al., 1991) and digoxigenin-labelled (Gemmrich et al., 1993) cDNA probes have been used for sensitive virus identification in RNA extracts from infected vines. Primers were designed for the specific amplification of viral sequences by RT-PCR (Rowhani et al., 1993), direct binding RT-PCR (Rowhani et al., 1995), and immunocapture RT-PCR (Brandt & Himmler, 1995) from different grapevine tissues (leaves, shoots, roots, and bark scrapings) and from viruliferous X. index extracts (Esmenjaud et al., 1994). RT-PCR detected as little as 128 fg of viral RNA and was estimated to be four to six fold more sensitive than ELISA (Rowhani et al., 1993, 1995; Brandt & Himmler, 1995).
Relationships
The virus is distantly related serologically to Arabis mosaic virus (ArMV) (Cadman et al., 1960; Barabino, 1963; Dias & Harrison, 1963; Martelli & Hewitt, 1963b; Taylor & Hewitt, 1964) and in nucleotide sequence (Fuchs et al., 1991). In cross protection tests, avirulent isolates protect to varying extents against virulent isolates of the same virus (Dias & Harrison, 1963; Taylor & Hewitt, 1964) or against isolates of ArMV (Huss et al., 1989).
Stability in Sap
In sap of herbaceous hosts the virus lost infectivity when heated for 10 min at 60-65 °C, diluted at 10-3-10-4 and stored for 2 to 4 weeks at 20 °C (Brückbauer & Rüdel, 1961b; Cadman et al., 1960; Dias, 1963; Martelli & Hewitt, 1963a; Vuittenez & Kuszala, 1963; Taylor & Hewitt, 1964).
Purification
The most common sources of virus for purification are infected plants of C. quinoa, G. globosa, P. vulgaris and N. clevelandii (Dias & Harrison, 1963; Martelli & Hewitt, 1963b; Vuittenez & Kuszala, 1963; Taylor & Hewitt, 1964; Quacquarelli et al., 1976; Pinck et al., 1988). The following two methods have been used successfully.
1. Grind frozen tissues directly in 1.5 vol of a 1:1 (v/v) mixture of chloroform-butan-l-ol with the addition of 1% (w/v) sodium ascorbate; centrifuge at 10,000 g for 10 min, collect the aqueous phase and subject it to two cycles of differential centrifugation. Resuspend pellets from high speed centrifugation in 0.01M phosphate buffer pH 7.2. Layer the final suspension on 10-40% sucrose density gradient columns and centrifuge for 2.5 h at 24,000 rpm in a Beckman SW 25.1 rotor. Collect the virus-containing fractions and sediment at 103,000 g for 2h (Quacquarelli et al., 1976).
2. Grind frozen leaves in 0.1M sodium phosphate buffer pH 7.2, 0.1M ascorbic acid, 0.01M EDTA, in the presence of 8.5% butanol. Clarify the extract at 10,000 g for 20 min and precipitate the virus by the addition of PEG (mol. wt 20,000) to 100 g/l and NaCl to 0.1M. Collect the precipitate by low speed centrifugation and resuspend in 0.2M sodium citrate pH 6.0. Subject the suspension to one cycle of differential centrifugation and resuspend pellets in 0.02M sodium citrate pH 6.0 containing 0.02% sodium azide. Centrifuge virus suspensions through a 20 ml cushion of 20% sucrose in 0.02M sodium citrate pH 6.0 at 35, 000 rpm for 5 h in a Beckman rotor 42. Resuspend pellets in 0.02M sodium citrate pH 6.0. (Pinck et al.,1988). Virus yield is generally low, ranging from 1 mg/400 g of G. globosa tissue (Martelli & Hewitt, 1963b) to 1 mg/200 g of French bean tissue (Taylor & Hewitt, 1964).
Properties of Particles
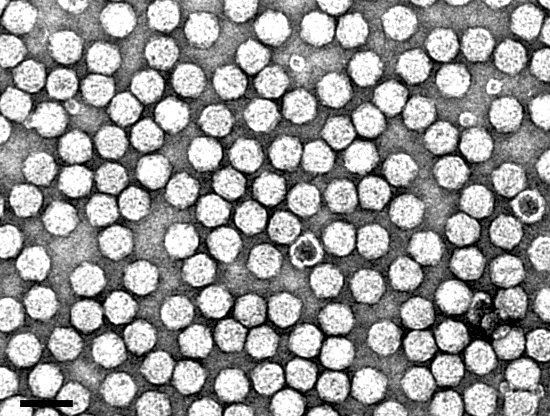
Virus particles are all of the same size (Fig. 11) but contain three density components that can be resolved in sucrose density gradients and in isopycnic centrifugation. These are empty protein shells (T), and two kinds of nucleoprotein particles with different amounts of RNA (M and B). The relative proportions of these components varies with the season of the year, with relative ratios of T:M:B in summer and winter being 1:0.5:3.5 and 1:0.5:0.75, respectively. B component is composed of two buoyant density species (Quacquarelli et al., 1976).
Sedimentation coefficient (s20,w): 50 S (T), 86 S (M), and126 S (B). (Quacquarelli et al., 1976).
Virus preparations centrifuged to equilibrium in CsCl yield bands of precipitated particles. Precipitation is not prevented by formaldehyde treatment prior to centrifugation. Buoyant density is: 1.31 (T), 1.41 (M), and 1.49 g/cm3 (B). (Quacquarelli et al., 1976).
Molecular weight: 3.3 x106 (T), 4.7 x 106 (M), 5.7 x 106 (B1), and 6.1 x 106 (B2) (Quacquarelli et al., 1976).
A260/A260: 0.73 (T), 1.50 (M), and 1.68 (B) (Quacquarelli et al., 1976).
Increment mid-point temperature (Tm) : 66 °C (Quacquarelli et al., 1976).
Isoelectric point: pH 4 (Hewitt et al., 1970).
Particle Structure
Virus particles are isometric, about 30 nm in diameter with an angular outline and poorly resolved surface structure (Martelli & Hewitt, 1963b) (Fig. 11). T particles are empty shells totally penetrated by the negative stain. The protein shell is constructed of 60 subunits with Mr of about 56,000 arranged in a T=1 icosahedral surface lattice (Quacquarelli et al., 1976).
Particle Composition
Nucleic acid. T particles do not contain nucleic acid. M and B components contain a single species of linear, positive sense, ssRNA (RNA-1 and RNA-2, respectively) accounting for about 30% (M) and 42% (B) of the weight of their particles (Quacquarelli et al., 1976). RNA-1 and RNA-2 have a mol. wt of about 2.4 x 106 and 1.4 x 106, respectively (Quacquarelli et al., 1976; Pinck et al., 1988). The weight proportion of each RNA species in RNA preparations from purified virus was calculated to be 26 ±3% (RNA-1) and 42 ±6% (RNA-2) (Pinck et al., 1988).
Protein. The viral coat protein accounts for about 70% of the weight of M particles and 58% of B particles and is composed of 60 subunits of a single polypeptide of Mr 54,000 as calculated by polyacrylamide gel electrophoresis (Quacquarelli et al., 1976) or of Mr 56,019, as deduced from the sequence of the coat protein gene (Serghini et al., 1990; Brandt et al., 1995).
Properties of Infective Nucleic Acid
Nucleic acid is readily extracted from purified virus particle preparations with Diener & Schneider's (1968) single-phase phenol/SDS method, or by freezing at -25 °C for 30 min and thawing, or by heating for 90 sec at 66 °C. Phenol/SDS extraction yields 40 to 60% of the theoretical maximum yield whereas higher yields are obtained with the other methods (Quacquarelli et al., 1976). RNA-2 is not infective. RNA-1 heated at 60 °C in 8M urea has a very low infectivity, but the relative infectivity of RNA-1 + RNA-2 is ten times higher, as determined by local lesion assay (Quacquarelli et al., 1976). Viral RNAs translated in rabbit reticulocyte lysates, direct the synthesis of two polyproteins with a Mr of 125,000 and 220,000, respectively (Morris-Krsinich et al., 1983), whereas proteins with a Mr of 127,000 and 225,000, respectively, were produced in wheat germ extracts (Pinck et al., 1988).
Genome Properties
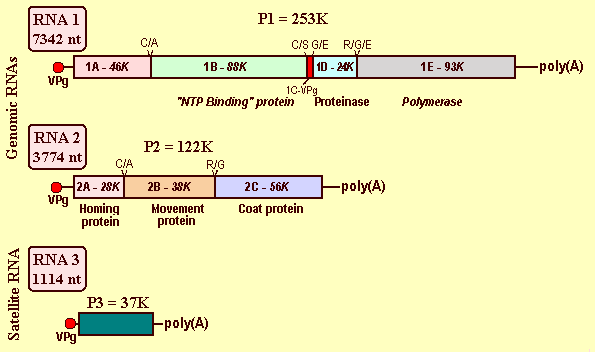
The viral genome is bipartite. Both RNAs are monocistronic, have a VPg composed of 24 residues (Mr 2.9K) at the 5' terminus (Pinck et al., 1988) and a poly(A) tail at the 3' terminus (Serghini et al., 1990; Ritzenthaler et al., 1991) (Fig. 12). The genome of the French F13 virus isolate (accessions X16907 and D00915) has been completely sequenced (Serghini et al., 1990; Ritzenthaler et al., 1991) and part of RNA-2 of an Austrian (Brandt & Himmler, 1995, accession U11768) and a Californian (Sanchez et al., 1991, accession X60775) isolate has also been sequenced. The strategy of expression is based on translation of both RNAs as polyproteins that are cleaved by an RNA-1 encoded viral proteinase (Pinck, 2000) (Fig. 12).
RNA-1 is 7,342 nt long and contains a single ORF of 6,855 nt, extending from nt 243 to nt 7097. The polyprotein encoded by this ORF is 2,284 amino acids in length with a Mr of 253,000. From the C- to the N-terminus, the primary structure of the polyprotein includes a putative RNA-dependent RNA polymerase 1E (Mr 92,000), followed by a cystein protease 1D (Mr 25,000) (Margis et al., 1991; Pinck, 1998), the 1C protein VPg (Mr 2900) (Pinck et al., 1991), a 1B protein (Mr 88,000) containing the signatures of a nucleotide binding domain and a protease cofactor (Ritzenthaler et al., 1991), and a N-terminal 1A protein (Mr 46,000) (Margis et al., 1994) (Fig. 12). Differential proteolytic activities of precursor and mature forms of the 1D proteinase at Arg/Gly, Cyst/Ala, and Gly/Glu cleavage sites have been identified (Margis et al., 1994).
RNA-2 is 3,774 nt long encoding a single product of Mr 131,000 (3330 nt). This polyprotein has three final in vitro maturation products (Ritzenthaler et al., 1995a). From the C- to the N-terminus, the primary structure of this polyprotein includes the 2C coat protein (CP), which is produced by proteolytic cleavage at the Arg/Gly site between residues 680 and 681 and contains 504 amino acids (Mr 56,019) (Serghini et al., 1990). Upstream of the CP is the 2B protein (Mr 38,000), formerly named P38, which is the putative movement protein that accumulates to very high concentrations in the cytosol of infected cells (Ritzenthaler et al., 1995a) and is also found in association with cell walls (Ritzenthaler et al., 1995b). The nine C-terminal residues of this protein are critical for systemic virus spread (Belin et al., 1999). The third maturation product is the N-terminal protein 2A (Mr 28,000) implicated in the replication of RNA-2 (Gaire et al., 1999) (Fig. 12).
Biologically active transcripts produced in vitro from full-length cDNA copies of RNA-1 and RNA-2, were infective when inoculated together to C. quinoa and were able to replicate and spread in plants producing symptoms. However, RNA-1, which replicates by itself in protoplasts, when inoculated alone to herbaceous hosts, could not be detected even in the inoculated leaves (Viry et al., 1993). An analysis of the genetic variability of virus isolates from infected vines in California has shown the quasi-species nature of the virus. Natural infections are made up of a mixture of closely related genomes, which are preferentially selected following passage through different alternative herbaceous hosts (Naraghi-Arani, 2000).
Satellite
The French virus isolate F13 supports the replication of a linear satellite RNA (satRNA) that is encapsidated, representing 66% of the RNA content of the virions (Pinck et al., 1988). The satRNA is 1,114 nt in length (accession D00442) and encodes a highly hydrophilic polypeptide of Mr 37,275. It has the same 5' and 3' terminal structures as the genomic RNAs (i.e. a VPg and a poly(A) tail) (Fig. 12). The coding sequence is preceded by a leader of 14 nt containing the consensus sequence U.G/UGAAAAU/AU/AU/A, which is also present in the genomic RNAs of the helper virus and several other nepoviruses, and is followed by a 3' terminal non coding region of 74 nt with no homology with its counterpart in genomic RNA species (Fuchs et al., 1989). The satRNA is unable to replicate on its own, requiring both RNAs of the helper virus for replication and encapsidation (Pinck et al., 1988; Hans et al., 1992). Its presence interferes with symptom expression in C. quinoa and decreases the amount of virus produced (Fuchs et al., 1991). The satRNA was detected by molecular hybridization in 5 of 34 virus isolates from different geographical locations (Saldarelli et al., 1993).
Relations with Cells and Tissues
The virus has been observed in the roots (Gerola et al., 1969) and mesophyll cells (Kalasjan et al, 1979) of grapevines, where particles are rare and aligned in short rows. In tissues of experimentally infected herbaceous hosts (C. quinoa, C. amaranticolor, N. clevelandii, P. hybrida) virus particles are much more abundant, being often arrayed in long parallel rows, looking like superimposed tubules aligned parallel to one another, although the particles do not appear to be enveloped (Gerola et al., 1969; Peña Iglesias & Rubio Huertos, 1971; Saric & Wrischer, 1975; Savino et al., 1985) (Fig. 13). True membranous tubules containing rows of particles are connected to plasmodesmata, or are present within cell wall protrusions that develop at the level of plasmodesmata. Aggregates of virus particles are common next to, or inside, large inclusion bodies (vesiculate-vacuolate inclusions) consisting of ribosomes, endoplasmic reticulum strands, and membranous vesicles containing fine fibrils (Gerola et al., 1969; Saric & Wrischer, 1975; Savino et al., 1985) (Fig. 14). Rows of empty particles are occasionally located in the nucleoplasm (Peña Iglesias & Rubio Huertos, 1971; Savino et al., 1985). Virus replicates in the cytoplasmic inclusion bodies whose membranous vesicles are thought to be the site of viral polyprotein processing and RNA replication (Pfeiffer et al., 2000).
Ecology and Control
In soils containing nematode vectors, infected vines occur typically in patches that enlarge at a rate of about 1 m/year (Hewitt et al., 1962). X. index is the major, most efficient and economically important vector. It has a limited range of alternative natural hosts (fig, mulberry, rose) that are immune to the virus. Infected seedlings and weeds (Lazar et al., 1990; Horvath et al., 1994) have limited epidemiological significance, although X. index was able to acquire the virus from the roots of C. amaranticolor and subsequently transmit it to V. rupestris (Hewitt et al., 1992). The virus persists in volunteer grapevine plants, and in the roots of lifted vines that remain viable in the soil and constitute an important source of virus inoculum (Hewitt et al., 1962; Taylor & Hewitt, 1964). It also persists for several months in the nematode vectors (Raski et al., 1965), whose vertical distribution in the soil follows closely that of the root system of the host (Lamberti & Martelli, 1965; Esmenjaud et al., 1992) and whose populations in temperate climates is not much affected by soil temperatures (Lamberti & Martelli, 1965). Controlling vectors by soil fumigation is difficult and seldom effective (Vuittenez, 1960; Hewitt et al., 1962). Cultural practices such as prolonged fallow, crop rotation, tillage, and weed control are equally of little effective (Taylor and Brown, 1997). Thus, local virus spread is difficult to restrain. By contrast, long distance spread can be readily controlled by the production and distribution of healthy propagative material (budwood, rooted rootstocks, or grafted vines). The virus can be eliminated by conventional (Galzy, 1961; Gifford & Hewitt, 1961; Goheen & Luhn, 1973), or modified (Stellmach, 1980; Monette, 1986) heat therapy, micrografting (Bass et al., 1978), and by in vitro meristem tip culture (Barlass et al., 1982).
Natural sources of resistance to the virus have been found in V. vinifera, V. munsoniana, and V. rotundifolia (Pantanelli, 1911; Walker et al., 1985). However, lack of infection of Muscadine grapes (V. munsoniana, V. rotundifolia) may result from graft incompatibility, rather than from true resistance (Walker et al., 1985; Bouquet,1981). In field assays, GFLV was not detected for nine years in V. vinifera x V. rotundifolia hybrid rootstocks (Walker et al., 1991) but, by the tenth year, high virus concentration was found in scions grafted onto these rootstocks (Walker et al., 1994).
Resistance to X. index feeding was identified in several Vitis species including V. rotundifolia (Boubals & Pistre, 1978), V. rufotomentosa (Harris, 1983), V. munsoniana (Staudt & Weischer, 1992) and some American rootstock hybrids (Kunde et al., 1968, Harris, 1983, Staudt & Kassemeyer, 1990). Muscadine grapes (V. rotundifolia) are highly resistant to virus transmitted by nematode feeding but not by graft-inoculation with infected scions (Bouquet, 1981). ELISA testing of roots was proposed for assessing resistance to virus transmission by X. index (Staudt, 1997).
Introducing pathogen-derived resistance into Vitis is being explored and the virus coat protein gene was engineered into rootstock and V. vinifera lines (Krastanova et al., 1995; Mauro et al., 1995; Gölles et al., 1997; Spielmann et al., 1997; Xue et al., 1997). Under experimental conditions, delayed infection or lower virus titre has been observed in some of the transgenic lines (Barbier et al., 1997; Courtois et al., 1997; Spielmann et al., 1997) but these results need to be evaluated in the field.
Notes
Several other nepoviruses infect grapevines, primarily in Central Europe (Germany and France), the Balkans, and North America (USA and Canada). Some of these viruses, such as Grapevine chrome mosaic virus, Arabis mosaic virus, Tomato black ring virus , and Tomato ringspot virus possess distorting and chromogenic strains that in Vitis induce symptoms very similar, if not indistinguishable, from those caused by GFLV (Martelli, 1993). The ecology and epidemiology of these viruses resembles that of GFLV except for the fact that they have alternative hosts on which nematode vectors (Xiphinema and Longidorus species) feed (Taylor & Brown, 1997).
Figures

Heavily deformed leaves with open petiolar sinuses and prominent marginal teeth from a vine infected by a distorting virus strain.

Green shoots from a vine infected by a distorting virus strain, showing internodes with irregular length and zigzag growth.

Severely deformed leaves with prominent teeth from a Vitis rupestris indicator plant chronically infected by a distorting virus strain. Healthy leaf on the left.

Differential reactions of Chenopodium quinoa infected by a severe virus strain (stunting and systemic mottling, on the left) and a mild virus strain (on the right.).

Distortion of the apical leaves of virus-infected Gomphrena globosa. These symptoms are highly characteristic of GFLV infections.
References list for DPV: Grapevine fanleaf virus (385)
- Baldacci, Belli, Betto & Refatti, Annali della Facoltà di Agraria dell' Università di Milano 10: 23, 1962.
- Barabino, Horticulture Research 3: 27, 1963.
- Barbier, Demangeat, Perrin, Cobanov, Jaquet & Walter, Extended Abstracts of the 12th Meeting of ICVG, Lisbon 1997: 131, 1997.
- Barlass, Skene, Woordam & Krake, Annals of Applied Biology 101: 291, 1982.
- Bass, Vuittenez & Legin, Proceedings of the 6th Meeting of ICVG, Cordoba 1976: 325, 1978.
- Belin, Schmitt, Gaire, Walter, Demangeat & Pinck, Journal of General Virology 80: 1347, 1999.
- Bercks & Querfurth, Phytopathologische Zeitschrift 65: 243, 1969.
- Boscia et al., in Sanitary Selection of the Grapevine. Protocols for the Detection of Viruses and Virus-like Diseases, p. 129, ed. B Walter, Les Colloques INRA n°86, Paris: INRA Editions, 1997.
- Boubals & Pistre, II Symposium International sur l'Amelioration de la Vigne, Bordeaux 1977: 199, 1978.
- Bouquet, Plant Disease 65: 791, 1981.
- Bovey, Gärtel, Hewitt, Martelli & Vuittenez, Virus and Virus-like Diseases of Grapevines, Lausanne: Editions Payot 181 pp, 1980.
- Brandt & Himmler, Vitis 34: 127, 1995.
- Brandt, Ibl & Himmler, Archives of Virology 140: 157, 1995.
- Brückbauer & Rüdel, Wein-Wissenschaft 16: 177, 1961a.
- Brückbauer & Rüdel, Wein-Wissenschaft 16: 197, 1961b.
- Cadman, Dias & Harrison, Nature, London 187: 577 1960.
- Catalano, Roca & Castellano, Nematologia Mediterranea 17: 13, 1989.
- Catalano, Savino & Lamberti, Proceedings of the 10th Meeting of ICVG, Volos 1990: 243,1991.
- Cholin, Mitteilung Weinbau Kellerwirtschaft 8: 63, 1896.
- Cohn, Tanne & Nitzany, Phytopathology 60: 181, 1970.
- Cory & Hewitt, Phytopathology 58:1316, 1968.
- Coste-Floret, Progres Agricole et Viticole 25: 683, 1896.
- Courtois, Gaire, Mauro, Toutain, Burrus, Pinck, Walter, Audran & Duteurtre, Extended Abstracts of the 12th Meeting of ICVG, Lisbon 1997: 133, 1997.
- Das & Raski, Nematologica 14: 55, 1968.
- Dias, 13° Congreso Luso-Espanol sobre Progreso Cientifico. 167, 1950.
- Dias, Annals of Applied Biology 51: 85, 1963.
- Dias & Harrison, Annals of Applied Biology 51: 97, 1963.
- Diener & Schneider, Archives of Biochemistry and Biophysics 124: 401, 1968.
- Esmenjaud, Walter, Valentin, Guo & Cluzeau, Agronomie 12: 395, 1992.
- Esmenjaud, Abad, Pinck & Walter, Plant Disease 78: 1087, 1994.
- Frantz & Walker, Vitis 34: 131, 1995.
- Fuchs, Pinck, Serghini, Ravelonandro, Walter & Pinck, Journal of General Virology 70: 955, 1989.
- Fuchs, Pinck, Etienne, Pinck & Walter, Phytopathology 81: 559, 1991a.
- Fuchs, Pinck, Serghini, Pinck & Walter, Proceedings of the 10th Meeting of ICVG, Volos 1990: 131, 1991b.
- Gaire, Schmitt, Stussi-Garaud, Pinck & Ritzenthaler, Virology 264: 25, 1999.
- Galzy, Comptes Rendus de l'Academie des Sciences, Paris 253: 706, 1961.
- Gemmrich, Link & Seidel, Vitis 32: 237, 1993.
- Gerola, Bassi & Belli, Giornale Botanico Italiano 103: 271, 1969.
- Gifford & Hewitt, American Journal of Enology and Viticulture 12: 129, 1961.
- Gifford, Hewitt, Graham & Lamoureux, Bulletin of the California Department of Agriculture 45: 268, 1956.
- Goheen & Luhn, Rivista di Patologia Vegetale 9: 287, 1973.
- Gölles, da Camara Machado, Minafra, Moser, Katinger & Laimer da Camara Machado, Extended Abstracts 12th Meeting of ICVG, Lisbon 1997: 139, 1997.
- Graniti & Russo, Proceedings of the International Conference on Virus and Vector on Perennial Hosts, with Special Reference to Vitis, Davis 1965: 261, 1965.
- Hans, Fuchs & Pinck, Journal of General Virology 73: 2517, 1992.
- Harris, Journal of Nematology 15: 415, 1983.
- Hewitt, Bulletin of the California Department of Agriculture 43: 47, 1954.
- Hewitt, Review of Applied Mycology 47: 433, 1968.
- Hewitt, Raski & Goheen, Phytopathology 48: 586, 1958.
- Hewitt, Goheen, Raski & Gooding, Vitis 3: 57, 1962.
- Hewitt, Martelli, Dias & Taylor, C.M.I./A.A.B. Descriptions of Plant Viruses 29, 1970.
- Hewitt, Goheen, Cory & Luhn, Annales de Phytopathologie, h.s.: 43, 1972.
- Horvath, Tobias & Hunyadi, Horticultural Science 26: 31, 1994.
- Huss, Walter, Etienne & Van Regenmortel, Vitis 25: 178, 1986.
- Huss, Müller, Sommermeyer, Walter & Van Regenmortel, Journal of Phytopathology 119: 358, 1987.
- Huss & Walter, Progres Agricole et Viticole 104: 275, 1987.
- Huss, Walter & Fuchs, Annals of Applied Biology 114: 45, 1989.
- Kalasjan, Litvak & Marinesku, Archives für Phytopathologie und Pflanzenschutz 15: 373, 1979.
- Krake & Woodham, Vitis 22: 40, 1983.
- Krastanova, Perrin, Barbier, Demangeat, Cornuet, Bardonnet, Otten, Pinck & Walter, Plant Cell Reporter 14: 550, 1995.
- Kunde, Lider & Schmitt, American Journal of Enology and Viticulture 19: 30, 1968.
- Lahogue, Boulard & Schneider, Vitis 34: 177, 1995.
- Lamberti & Martelli, Proceedings of the International Conference on Virus and Vector on Perennial Hosts, with Special Reference to Vitis, Davis 1965: 353, 1965.
- Lazar, Kölber & Lehoczky, Kergazdasag 22: 58, 1990.
- Margis, Viry, Pinck & Pinck, Virology 185: 779, 1991.
- Margis, Viry, Pinck, Bardonnet & Pinck, Virology 200: 79, 1994.
- Martelli G. P. (ed.), Graft-transmissible Diseases of Grapevines. Handbook for Detection and Diagnosis, Rome: FAO Publication Division, 263 pp, 1993.
- Martelli & Hewitt, Phytopathologia Mediterranea 2: 275, 1963a.
- Martelli & Hewitt, Phytopathologia Mediterranea 2: 285, 1963b.
- Mauro, Toutain, Walter, Pinck, Otten, Coutos-Thevenot, Deloire & Barbier, Plant Science 112 : 97, 1995.
- Monette, Journal of Phytopathology 116: 88, 1986.
- Morris-Krsinich, Forster & Mossop, Virology 130: 523, 1983.
- Naraghi-Arani, Ph.D. Thesis, University of California, Davis, 2000.
- Pantanelli, Rendiconti Regia Accademia dei Lincei 19 (S.V. I sem): 395, 1910.
- Pantanelli, Rendiconti Regia Accademia dei Lincei 20 (S.V. I sem): 575, 1911.
- Peña Iglesias & Rubio Huertos, Microbiologia Española 24: 184, 1971.
- Petri, Rendiconti Regia Accademia dei Lincei 22 (S.V, II sem): 154, 1913.
- Petri, Rendiconti Regia Accademia dei Lincei 27 (S.V, II sem): 271, 1918.
- Pfeiffer, Ritzenthaler, Gaire, Schmitt, Rohfritsch, Laporte, Pinck & Stussi-Garaud, Extended Abstracts of the 13th Meeting of ICVG, Adelaide 2000: 63, 2000.
- Pinck, in Handbook of Proteolytic Enzymes, p.719, eds A. J. Barrett & J. F. Woesser, Academic Press, London, 1998.
- Pinck, Extended Abstracts 13th Meeting of ICVG, Adelaide 2000: 60, 2000.
- Pinck, Fuchs, Pinck, Ravelonandro & Walter, Journal of General Virology 69: 233, 1988.
- Pinck, Reinbolt, Loudes, Le Ret & Pinck, FEBS Letters 284: 117, 1991.
- Quacquarelli, Gallitelli, Savino & Martelli, Journal of General Virology 32: 349, 1976.
- Raski, Hewitt, Goheen, Taylor & Taylor, Nematologica 11: 349, 1965.
- Raski, Maggenti & Jones, Journal of. Nematology 5: 208, 1973.
- Rathay, Österreich Botanische Zeitschrift 32: 316, 1882.
- Ritzenthaler, Viry, Pinck, Margis, Fuchs & Pinck, Journal of General Virology 72: 2357, 1991.
- Ritzenthaler, Schmit, Michler, Stussi-Garaud & Pinck, Molecular Plant Microbe Interactions 8: 379, 1995a.
- Ritzenthaler, Pinck & Pinck, Journal of General Virology 76: 907, 1995b.
- Rowhani, Walker & Rokni, Vitis 31: 35, 1992.
- Rowhani, Chay, Golino & Falk, Phytopathology 83: 749, 1993.
- Rowhani, Maningas, Lile, Daubert & Golino, Phytopathology 85: 347, 1995.
- Russo, Martelli & Savino, Proceedings of the 7th Meeting of ICVG, Niagara Falls 1980, Canada Agriculture, Research Branch: 251, 1980.
- Saldarelli, Minafra & Walter, Vitis 32: 99, 1993.
- Sanchez, Chay, Borja, Rowhani, Romero, Bruening & Ponz, Nucleic Acids Research 19: 5440, 1991.
- Saric & Wrischer, Phytopathologische Zeitschrift 84: 97, 1975.
- Savino, Cherif & Martelli, Phytopathologia Mediterranea 24: 29, 1985.
- Serghini, Fuchs, Pinck, Reinbolt, Walter & Pinck, Journal of General Virology 71: 1433, 1990.
- Spielmann, Krastanova, Duet-Ohrant, Marc-Martin, Prince Signrist & Gugerli, Extended Abstracts of the 12th Meeting of ICVG, Lisbon 1997: 143, 1997.
- Staudt, Vitis 36 : 155, 1997.
- Staudt & Kassemeyer, Proceedings of the 5th International Symposium on Grape Breeding, St. Martin/Pfalz 1989: 223, 1990.
- Staudt & Weischer, Wein-Wiss.enschaft 47: 56, 1992.
- Stellmach, Proceedings of the 7th Meeting of ICVG, Niagara Falls 1980, Canada Agriculture, Research Branch: 325, 1980.
- Szychowski, McKenry, Walker, Wolpert, Credi & Semancik, Vitis 34: 229, 1995.
- Taylor & Brown, Nematode Vectors of Plant Viruses, Wallingford: CAB International, 286 pp, 1997.
- Taylor & Hewitt, Journal of Agricultural Research, 15: 571, 1964.
- Taylor & Robertson, Annals of Applied Biology 66: 373, 1970.
- Viala, Les Maladies de la Vigne, Paris: George Masson, 1893.
- Viry, Serghini, Hans, Ritzenthaler, Pinck & Pinck, Journal of General Virology 74: 169, 1993.
- Vuittenez, Comptes Rendues Hebdomadaires des Seances de l'Academie Agricole de France 20: 89, 1960.
- Vuittenez, Comptes Rendues Hebdomadaires des Seances de l'Academie Agricole de France 49: 795, 1963.
- Vuittenez & Kuszala, Etudes de Virolologie Appliqué 4: 133, 1963.
- Walker, Meredith & Goheen, Vitis 24: 218, 1985.
- Walker, Lider, Goheen & Olmo, HortScience 26: 1224, 1991.
- Walker, Wolpert & Weber, Vitis 33: 19, 1994.
- Walter & Etienne, Journal of Phytopathology 120: 355, 1987.
- Walter & Martelli, Bulletin de l' O.I.V. 69: 945, 1996.
- Walter, Vuittenez, Kuszala, Stocky, Burckard & Van Regenmortel, Agronomie 4: 527, 1984.
- Xue, Krastanova, Ling, Sekya, Zhu, Petrovic, Reid, Velasquez, Burr & Gonsalves, Extended Abstracts of the 12th Meeting of ICVG, Lisbon 1997: 137, 1997.