Details of DPV and References
DPV NO: 398 July 2003
Family: Virgaviridae
Genus: Tobravirus
Species: Tobacco rattle virus | Acronym: TRV
This is a revised version of DPV 346
Tobacco rattle virus
D. J. Robinson Scottish Crop Research Institute, Invergowrie, Dundee, DD2 5DA, UK
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Mauche disease of tobacco was described by Behrens (1899), and the causal virus by Quanjer (1943).
- Selected synonyms:
- Aster ringspot virus
(Anderson, 1954)
- Potato corky ringspot virus (Eddins et al., 1946)
- Potato stem mottle virus (stengelbont virus) (Rozendaal, 1947)
- Ratel virus (Quanjer, 1943)
- Tabakmauche Virus (Behrens, 1899)
- Tabak Streifen und Kräuselkrankheit Virus (Böning, 1928)
- Potato corky ringspot virus (Eddins et al., 1946)
A virus with straight tubular particles of two predominant lengths, the longer about 190 nm and the shorter 50 to 115 nm, depending on the isolate. Normal particle-producing isolates (called M-type) have two species of genomic RNA, RNA-1 and RNA-2, are readily transmitted by inoculation with sap, and by nematodes in the family Trichodoridae. Other isolates (called NM-type) have only RNA-1, do not produce particles, are transmitted with difficulty by inoculation with sap, and are probably not transmitted by nematodes. The virus has a wide host range including many cultivated species, and occurs in several parts of the world.
Main Diseases
In potato, it causes one type of necrotic arcing, known as spraing, corky ringspot, Pfropfenbildung or kringerigheid, in the tuber flesh (Fig. 1). In plants grown from affected tubers, one or a few of the shoots may be distorted or stunted and may bear leaves that show a mottle or mosaic (Fig. 2), a condition referred to as stem mottle (Harrison & Robinson, 1981). However, systemic infection is rarely complete and spraing-affected tubers may give rise to virus-free progeny plants. In some cultivars, systemic infection may be complete and the virus may be passed on to most or all of the vegetative progeny, but few if any symptoms are produced in the tubers (Xenophontos et al., 1998). Other diseases caused include: notched leaf in gladiolus (Cremer & Schenk, 1967); malaria (necrotic spots in the bulb flesh) in hyacinth (Van Slogteren, 1958); ringspot in aster (Anderson, 1954); yellow blotch in sugar beet (Gibbs & Harrison, 1964); yellow mottle in spinach (Bailiss & Okonkwo, 1979); rattle (systemic necrotic flecks and line patterns, and death or stunting of shoots; also known as Mauche, ratel, Streifen und Kräuselkrankheit) in tobacco (Behrens, 1899) (Fig. 3; Fig. 4); and unnamed diseases in lettuce, hydrangea, narcissus, tulip (Fig. 5) and several other ornamental species. Many weed species (Fig. 6) become infected in nature, especially in their roots, but several are invaded systemically and some of these, such as Stellaria media, may show no obvious symptoms.
Geographical Distribution
Europe, Japan, New Zealand, North America.
Host Range and Symptomatology
The host range is very wide. More than 400 species in more than 50 dicotyledonous and monocotyledonous families can be infected experimentally; in many instances the infection does not become systemic (Uschdraweit & Valentin, 1956; Noordam, 1956; Schmelzer, 1957; Horváth, 1978).
- Diagnostic species
Chenopodium amaranticolor. Necrotic local lesions (Fig. 7), sometimes tending to spread, develop 3-5 days after inoculation; most virus isolates do not become systemic.
Chenopodium quinoa. Chlorotic or necrotic local lesions, usually spreading, develop 3-4 days after infection; infection often spreads into the petioles, causing the inoculated leaves to collapse, but rarely spreads further.
Nicotiana tabacum cv. Samsun NN (tobacco). Typical symptoms include necrotic spots or rings in inoculated leaves, and sporadic systemic distortion and/or necrotic line patterns. However, symptoms depend on the environmental conditions, and some virus strains produce other symptoms, or none at all.
Phaseolus vulgaris (French bean). Pin-point, necrotic local lesions develop 2-4 days after inoculation (Fig. 8); not systemic.
Pisum sativum (pea) and Vicia faba (broad bean). Small necrotic local lesions; not systemic.
- Propagation species
Nicotiana clevelandii, which becomes infected systemically, is the best host for maintaining cultures and as a source of virus for purification.
- Assay species
Chenopodium amaranticolor, and primary leaves of Phaseolus vulgaris, are suitable for local lesion assay.
Petunia hybrida, Nicotiana tabacum cv. White Burley and Cucumis sativus are useful bait plants for testing transmission by vectors; infection of the bait plants is best determined by assaying the infectivity of root extracts.
Strains
Many strains are described but few can be reliably distinguished by symptoms in test plants. However, symptomatologically distinguishable variants can often be isolated from a bulk culture (Cadman & Harrison, 1959). Some of the best characterized strains are:
PRN (potato ring necrosis; Cadman & Harrison, 1959; Harrison & Nixon, 1959). Originally obtained from potato in Scotland. Now used as the type strain, although it seems to have lost the ability to be transmitted by nematodes (Ploeg et al., 1992a). The short particles are 78 nm long.
PpK20 (Ploeg et al., 1992b). Originally obtained by transmission from a single Paratrichodorus pachydermus from Scotland. Serologically closely related to PRN.
SYM (spinach yellow mottle; Kurppa et al., 1981). Originally obtained from spinach in England. Causes systemic distortion and necrosis in Chenopodium amaranticolor unless dilute inocula are used. The short particles are 101 nm long.
Oregon strains. Originally obtained from potato in Oregon (Allen, 1963), and separated and characterized by Lister & Bracker (1969). One variant (Oregon Yellow) induces yellow ringspots and line patterns in N. clevelandii and N. glutinosa; Oregon Severe causes severe systemic necrosis in these hosts, and Oregon Mild causes only mild symptoms. The short particles are 90, 100 or 81nm long, respectively.
Italian No. 6 (Van Hoof et al., 1966). Originally obtained from tobacco bait seedlings planted in soil from Italy. Serologically related to and classified as Pea early-browning virus (English serotype) by Harrison (1973), but re-assigned as TRV by Robinson et al. (1987). The short particles are 105 nm long.
N5 (Harrison et al., 1983). Originally obtained from narcissus in Scotland. Causes severe necrosis in N. clevelandii and kills many of the plants. Serologically closely related to the Dutch serotype of Pea early-browning virus (Robinson et al., 1987). The short particles are 75 nm long.
PSG, PLB and TCM. Originally obtained from potato (PSG and PLB) or tulip (TCM) in the Netherlands (Cornelissen et al., 1986; Angenent et al., 1989). TCM is serologically related to the Dutch serotype of Pea early-browning virus.
Hypochoeris mosaic virus, previously listed as a tentative member of the genus Furovirus, has been shown to be serologically related to TRV and, although the original isolate no longer exists, it was probably a strain of TRV (Uhde et al., 1998).
Isolates unable to produce nucleoprotein particles (NM-type isolates) can be obtained from any of the above strains by using inocula containing only long particles. They are poorly transmissible by mechanical inoculation with sap but more easily transmitted by using nucleic acid inocula made with the aid of phenol (Sänger & Brandenburg, 1961). Many of them cause more necrosis in plants than do their parent M-type cultures (Fig. 9) and they are slower to become systemic (Cadman, 1962). NM-type isolates are also found in naturally infected plants.
Transmission by Vectors
At least 12 nematode species in the genera Paratrichodorus and Trichodorus (Trichodoridae) (Fig. 10) are natural vectors in Europe, N. America or Japan (Taylor & Brown, 1997). There is considerable specificity between virus strain and vector species (Ploeg et al., 1992a, 1992b); in general, a given virus isolate can be transmitted by only one or a few species of trichodorid, and each vector species can transmit only virus isolates belonging to one or a few serotypes. Adults and juveniles can transmit, but virus is probably not retained through the moult. The virus can be acquired by P. allius in 1 h, inoculated in 1 h (Ayala & Allen, 1968) and retained for many months by non-feeding nematodes (Van Hoof, 1970). The virus particles become attached to the oesophageal wall of the nematodes (Taylor & Robertson, 1970, Fig. 11) and are thought to be egested with saliva into root cells probably during exploratory probes that do not kill the target cell. There is no evidence for multiplication of the virus in the vector and it is probably not transmitted through nematode eggs (Ayala & Allen, 1968).
Transmission through Seed
Up to 10% seed transmission in Viola arvensis, and lower frequencies in some other weeds (Lister & Murant, 1967; Cooper & Harrison, 1973). Probably not transmitted through true potato seed (Dale et al., 2000).
Transmission by Dodder
At least six Cuscuta spp. can transmit and the virus infects the dodder (Schmelzer, 1956).
Serology
Several strains are poorly immunogenic but antisera with tube precipitin titres of 1/1000 have been made against others. In tube precipitin tests, the precipitates are intermediate between somatic and flagellar in type. Immunosorbent electron microscopy and ELISA (Harrison et al., 1983) are now the most used tests. Double diffusion tests in agar or agarose gel are insensitive. Monoclonal antibodies to strain PLB have been prepared (Legorburu et al., 1995).
Relationships
TRV has similar particle and genome properties to other tobraviruses. Indeed, distant serological relationships to Pea early-browning virus (Maat, 1963) and Pepper ringspot virus (Harrison & Woods, 1966; Kurppa et al., 1981) have been reported, but these viruses do not form pseudo-recombinants with TRV (Frost et al., 1967; Lister, 1968; Robinson & Harrison, 1985b), and there is only limited nucleotide sequence identity (58-65%) between the RNA-1 species of the three viruses (MacFarlane, 1999). In contrast, the nucleotide sequences of RNA-1 of three isolates of TRV are >99% identical, and all isolates cross-react in nucleic acid hybridization tests with probes for RNA-l (Robinson & Harrison, 1985a; Robinson et al., 1987). Pseudo-recombinant isolates are readily produced with all combinations of RNA-1 and RNA-2 from different TRV strains (Harrison & Robinson, 1986). However, different strains of TRV have very little nucleotide sequence identity in RNA-2 (Robinson & Harrison, 1985a; MacFarlane, 1999), and this is reflected in the existence of many serotypes (Harrison & Woods, 1966; Robinson & Harrison, 1985a). Members of different serotypes are only distantly related serologically, and there is also evidence for serological variation within serotypes. Some strains, such as Italian No. 6 and N5, are closely related serologically to one or other of two serotypes of Pea early-browning virus, but are classified as TRV on the basis of nucleic acid hybridization tests with RNA-1 probes and pseudo-recombination tests (Robinson et al., 1987).
Stability in Sap
In infected N. clevelandii sap, the thermal inactivation point (10 min) of M-type isolates is 80-85°C, the dilution end-point is 10-5 to 10-6, and infectivity is retained at 20°C for more than 6 weeks and at -20°C for many years. By contrast, NM-type isolates lose infectivity when sap is heated for 10 min at 50°C, diluted to 10-1, kept at 20°C for 1 h or frozen at -20°C (Cadman & Harrison, 1959; Cadman, 1962).
Purification
Systemically infected N. clevelandii leaves yield 10-100 mg virus particles per kg leaf by either of the following methods of purification.
1. Harrison & Nixon, 1959; Robinson, 1983. Store extracted sap at -20°C. Thaw the sap, clarify by low-speed centrifugation and precipitate virus particles with 10% (w/v) polyethylene glycol Mol. Wt 6000 and 2% (w/v) NaCl. Allow the pellets to resuspend in 70 mM phosphate buffer (pH 7.5) for 16 h at 4°C, and further purify virus particles by two cycles of differential centrifugation.
2. Lister & Bracker, 1969. Grind cooled leaves in 10 mM citric acid + phosphate buffer (pH 7.4), containing 0.1% sodium thioglycollate. Clarify the extract by blending with 0.5 vol. of a 1:1 mixture of butan-1-ol and chloroform and freeze the aqueous layer overnight. Thaw the extract and purify virus particles by differential centrifugation, resuspending pellets in 10 mM phosphate buffer (pH 7.0).
Properties of Particles
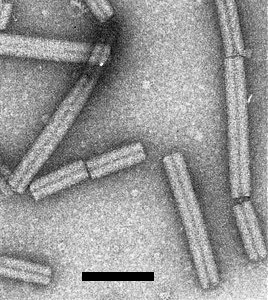
The tubular virus particles are of two predominant lengths (Fig. 12), the longer ones (L) 185-196 nm and the shorter ones (S) ranging from 50 to 115 nm, depending on the isolate (Harrison & Robinson, 1978). L and S particles, which contain RNA-1 and RNA-2 respectively, can be separated by sedimentation in sucrose density gradients. L particles are infective alone and induce the synthesis of RNA-1 but not of virus particles. S particles are not infective alone but carry the gene for the virus particle protein. L and S particles are produced when the inoculum contains them both (Lister, 1966; Frost et al., 1967; Lister & Bracker, 1969; Sänger, 1969). Many virus strains produce in addition small amounts of shorter particles that contain subgenomic RNA species (Robinson et al., 1983).
Sedimentation coefficients (s20,w; svedbergs): 296-306 (L), 155-245 (S) (Harrison & Woods, 1966; Semancik, 1966).
Buoyant density (g.cm-3) in CsCl: 1.324 (Cooper & Mayo, 1972).
Particle weight (daltons x 106): 48-50 (L) and 11-29 (S) (Harrison & Klug, 1966).
Electrophoretic mobility (cm2 sec-1 V-1): -1.7 x 10-5 (strain PRN, I = 0.15, pH 7.0) (Harrison & Nixon, 1959); -1.1 x 10-5 (strains B and C, I = 0.1, pH 7.0) (Semancik, 1966).
Extinction coefficient (A260nm, 0.1%, 1cm): about 3.0 (Lister & Bracker, 1969).
A260/A280: 1.10-1.15 (Harrison & Nixon, 1959).
Particle Structure
Particles are tubular with helical symmetry, 23 nm in diameter with a central canal of diameter about 5 nm (Nixon & Harrison, 1959; Cooper & Mayo, 1972). The number of subunits per turn = n + 1/3. Alternative values proposed for n are 25 (Finch, 1965), or about 32 (Roberts & Mayo, 1980). Pitch = 2.5 nm (Finch, 1965). A model of the protein subunit structure proposed by Goulden et al. (1992) is supported in part by Raman optical activity measurements (Blanch et al., 2001). The C-terminal portion of the coat protein is exposed on the surface of particles (Mayo et al., 1993) and is antigenically dominant (Legorburu et al., 1996).
Particle Composition
Nucleic acid: RNA, single-stranded, linear; two genomic species, RNA-1 and RNA-2, packaged separately in L and S particles respectively, and comprising 5% of the particle weight. RNA-l comprises 6790 or 6791 nucleotides (Mol. Wt 2.2 x 106) (Hamilton et al., 1987; Sudarshana & Berger, 1998), whereas RNA-2 ranges from 1905 to 3926 nucleotides (Mol. Wt 0.6 to 1.3 x 106), depending on the strain (MacFarlane, 1999). Two subgenomic RNA species are packaged in separate particles: RNA-1a, derived from RNA-1, Mol. Wt 0.54 x 106 in several strains; RNA-2a, derived from RNA-2, Mol. Wt 0.6 x 106 in strain SYM, but of different size in other strains (Robinson et al., 1983, 1987). Subgenomic RNA-1b, derived from the 3' terminal region of RNA-1, does not become packaged in virus particles (Boccara et al., 1986).
Protein: 95% of particle weight. One species of Mol. Wt 21.5 to 22.9 x 103, depending on the strain. On storage of virus particles in the absence of antimicrobial agents, the coat protein is partially degraded to a lower Mol. Wt form (Mayo & Cooper, 1973).
Genome Properties
Accession numbers for nucleotide sequences of complete genome parts of various strains are:
RNA-1: D00155 (SYM), AF034622 (ORY), AF314165 (PpK20)
RNA-2: J04347 (PLB), X03686 (PSG), X03955 (TCM), Z36974 (PpK20), Z97357 (ON), AF034621 (ORY), AJ007293 (SP), AJ009833 (TpO1), AJ250488 (PaY4), AJ272198 (Rostock).
Partial sequences from several other strains are also available in the database.
5' termini are probably capped (Pelham, 1979), and 3' termini have tRNA-like secondary structures including a pseudoknot, although no amino acid acceptor activity was detected (Van Belkum et al., 1987). There is no genome-linked protein or polyadenylate terminal sequence.
RNA-1 is capable of independent replication and systemic spread in plants. It carries genetic determinants for lesion type and for systemic infection in Nicotiana spp. (Cadman & Harrison, 1959; Sänger, 1969). It contains four open reading frames (ORFs; Fig. 13), coding for (from the 5' end): a 134 K protein that contains amino acid motifs associated with methyl transferase and helicase proteins and is terminated by an opal stop codon; a 194 K protein, produced by read-through of this stop codon (Pelham, 1979; Zerfass & Beier, 1992), which has motifs typical of RNA-dependent RNA polymerases; protein 1a, a 29 K cell-to-cell movement protein (Ziegler-Graff et al., 1991); and protein 1b (16 K), which may be a suppressor of post-transcriptional gene silencing (Liu et al., 2002).
RNA-2 carries genetic determinants, depending on the virus strain, for the ability to cause systemic symptoms in Chenopodium amaranticolor (Kurppa et al., 1981), for the ability to cause yellow mosaic in tobacco (Lister & Bracker, 1969), for serological specificity (Sänger, 1969) and for vector transmissibility (Ploeg et al., 1993). The coding capacity of RNA-2 in different strains is quite diverse, but all can be regarded as variants of the basic type, represented by strain PpK20 (Fig. 13). This basic version of RNA-2 contains three ORFs, coding for (from the 5' end): the coat protein; protein 2b (27K to 40K in different strains), which is absolutely required for transmission by nematodes (Hernández et al., 1997); and protein 2c (18K to 33K in different strains) of unknown function. Variations on this basic arrangement are of three types. First, some strains contain an additional ORF, potentially coding for a 9K protein, between the genes for coat protein and protein 2b; there is no evidence that this ORF is expressed. Second, the 2c gene or the 2b and 2c genes may be missing wholly or in part; such deletions are probably associated with loss of transmissibility by nematodes. Third, whereas sequences at both ends of RNA-2 are nearly identical to those at the ends of RNA-1, the extent of this region of identity varies between strains; in some strains the RNA-1-like region at the 3' end of RNA-2 is large enough to include some or all of the protein 1b ORF and even part of the protein 1a ORF (Cornelissen et al., 1986; Angenent et al., 1986; Robinson et al., 1987; Angenent et al., 1989).
In vitro, and presumably in vivo, the 134 K and 194 K proteins are translated from RNA-1 (Fritsch et al., 1977), whereas proteins 1a, 1b and the coat protein are translated from subgenomic RNA species 1a, 1b and 2a, respectively (Robinson et al., 1983; Boccara et al., 1986; Cornelissen et al., 1986). The means by which proteins 2b and 2c are produced are unknown, nor is it known whether the duplicate copy of the protein 1b ORF on RNA-2 of some strains is expressed.
Relations with Cells and Tissues
Most tissues of systemically invaded plants become infected. In leaf hair cells of N. clevelandii, `X-bodies' largely composed of abnormal mitochondria can be induced by strain PRN, but persist for only a few days. Small aggregates of virus particles occur in the X-bodies and elsewhere in the cytoplasm (Harrison et al., 1970).
Ecology and Control
TRV is essentially a virus of weeds and other wild plants (Noordam, 1956; Cooper & Harrison, 1973), and its distribution in soil reflects that of its viruliferous vectors, which prefer light sandy or peaty soils (Cooper, 1971). It may be patchily distributed within a field. Control measures include the use of nematicidal and nematostatic chemicals, and the planting of resistant cultivars or virus-free stock (Harrison & Robinson, 1986). However, it should be noted that, in potato, although there are cultivars that are resistant to infection, others are merely tolerant, i.e. they may be systemically infected but produce few or no symptoms (Xenophontos et al., 1998). Infected plants of such cultivars are difficult to recognize but can act as sources for acquisition of virus by vector nematodes, and thus if used as seed tubers may spread the virus to new sites.
Notes
TRV is distinguished from viruses in other genera by its characteristic particles, together with its symptoms in diagnostic hosts and ability to produce NM-type isolates. Strain CAM and other South American isolates, previously classified as TRV serotype III, are now considered to be isolates of Pepper ringspot virus (Robinson & Harrison, 1985a). Pepper ringspot virus is at best only distantly related serologically to TRV, there is no cross-reaction in nucleic acid hybridization tests with RNA-1 probes (Robinson & Harrison, 1985a), and no amplification is observed in RT-PCR with TRV-specific primers (Robinson, 1992). TRV can be distinguished from Pea early-browning virus by failure to infect pea or Phaseolus vulgaris systemically, by production of pinpoint lesions in P. vulgaris, by nucleic acid hybridization tests using RNA-1 probes, and by RT-PCR (Robinson, 1992). M-type isolates of TRV can sometimes be identified by serological tests but the great antigenic differences between strains make negative results in such tests inconclusive. However, all isolates of both M- and NM-types can be detected by nucleic acid hybridization tests with RNA-1 probes (Robinson & Harrison, 1985a; Robinson et al., 1987) or by RT-PCR (Robinson, 1992).
Figures
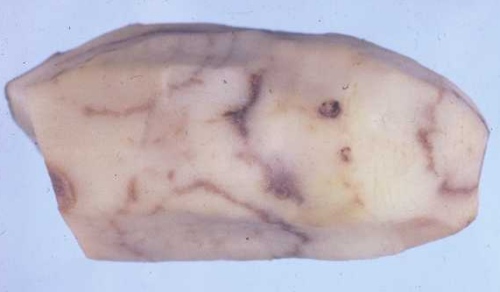
Tuber of naturally infected potato (Solanum tuberosum cv. Pentland Dell) cut to show necrotic arcs in the flesh (spraing). Photograph courtesy of the Scottish Crop Research Institute.

Mosaic in a leaf of naturally infected potato (Solanum tuberosum cv. Majestic). Photograph courtesy of the Scottish Crop Research Institute.

Necrotic symptoms in a naturally infected tobacco (Nicotiana tabacum) plant. Photograph courtesy of F Bem, Benaki Phytopathological Institute.

Necrotic flecks and line patterns in a systemically infected leaf of tobacco (Nicotiana tabacum). Photograph courtesy of F Bem, Benaki Phytopathological Institute.

Dark streaks in petals of infected tulip cv. Apeldoorn. Photograph courtesy of the Scottish Crop Research Institute.
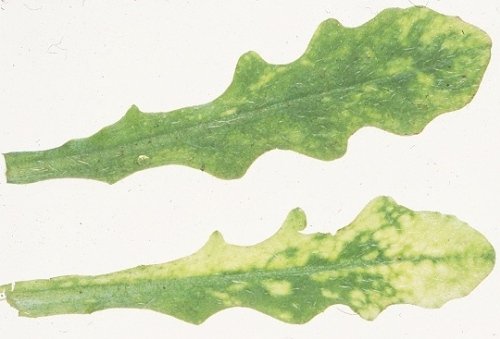
Symptoms in leaves of a naturally infected weed (Hieracium sp.). Photograph courtesy of the Scottish Crop Research Institute.

Necrotic local lesions in an inoculated leaf of Chenopodium amaranticolor. Photograph courtesy of the Scottish Crop Research Institute.

Necrotic local lesions in a leaf of Phaseolus vulgaris inoculated with strain PRN. Photograph courtesy of the Scottish Crop Research Institute.

Nicotiana clevelandii plants systemically infected with (left) M-type and (right) NM-type isolates of strain SYM. (From Harrison & Robinson, 1981).
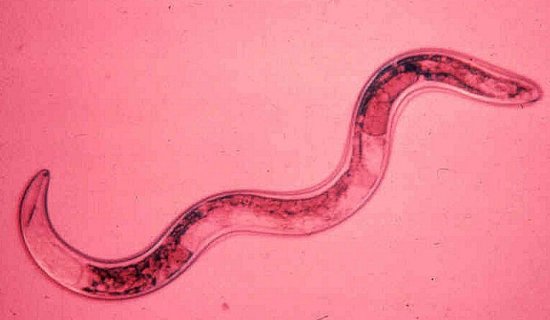
Adult female Paratrichodorus pachydermus. Photograph courtesy of the Scottish Crop Research Institute.
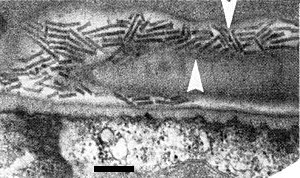
Virus particles in a longitudinal chordal section of the oesophagus of Paratrichodorus pachydermus. Arrows indicate the oesophageal walls. Bar represents 200 nm. (From Taylor & Robertson, 1970).
References list for DPV: Tobacco rattle virus (398)
- Allen, Plant Disease Reporter 47: 920, 1963.
- Anderson, Phytopathology 44: 87, 1954.
- Angenent, Linthorst, Van Belkum, Cornelissen & Bol, Nucleic Acids Research 14: 4673, 1986.
- Angenent, Posthumus, Brederode & Bol, Virology 171: 271, 1989.
- Ayala & Allen, Journal of Agriculture of the University of Puerto Rico 52: 101, 1968.
- Bailiss & Okonkwo, Journal of Horticultural Science 54: 289, 1979.
- Behrens, Landwirtschaftlichen Versuchsstationen 52: 422, 1899.
- Blanch, Robinson, Hecht & Barron, Journal of General Virology 82: 1499, 2001.
- Boccara, Hamilton & Baulcombe, EMBO Journal 5: 223, 1986.
- Böning, Arbeiten aus der Beyerischen Landesanstalt für Pflanzenbau und Pflanzenschutz 4: 1928.
- Cadman, Nature, London 193: 49, 1962.
- Cadman & Harrison, Annals of Applied Biology 47: 542, 1959.
- Cooper, Plant Pathology 20: 51, 1971.
- Cooper & Harrison, Annals of Applied Biology 73: 53, 1973.
- Cooper & Mayo, Journal of General Virology 16: 285, 1972.
- Cornelissen, Linthorst, Brederode & Bol, Nucleic Acids Research 14: 2157, 1986.
- Cremer & Schenk, Netherlands Journal of Plant Pathology 73: 33, 1967.
- Dale, Robinson, Griffiths, Todd & Bain, European Journal of Plant Pathology 106: 275, 2000.
- Eddins, Proctor & West, American Potato Journal 23: 330, 1946.
- Finch, Journal of Molecular Biology 12: 612, 1965.
- Fritsch, Mayo & Hirth, Virology 77: 722, 1977.
- Frost, Harrison & Woods, Journal of General Virology 1: 57, 1967.
- Gibbs & Harrison, Plant Pathology 13: 144, 1964.
- Goulden, Davies, Wood & Lomonossoff, Journal of Molecular Biology 227: 1, 1992.
- Hamilton, Boccara, Robinson & Baulcombe, Journal of General Virology 68: 2563, 1987.
- Harrison, CMI/AAB Descriptions of Plant Viruses 120: 1973.
- Harrison & Klug, Virology 30: 738, 1966.
- Harrison & Nixon, Journal of General Microbiology 21: 569, 1959.
- Harrison & Robinson, Advances in Virus Research 23: 25, 1978.
- Harrison & Robinson, in Handbook of Plant Virus Infections and Comparative Diagnosis, p. 515, ed. Kurstak, Amsterdam: Elsevier/North-Holland, 1981.
- Harrison & Robinson, in The Plant Viruses, vol. 2, p. 339, eds Van Regenmortel & Fraenkel-Conrat, New York: Plenum, 1986.
- Harrison & Woods, Virology 28: 610, 1966.
- Harrison, Stefanac & Roberts, Journal of General Virology 6: 127, 1970.
- Harrison, Robinson, Mowat & Duncan, Annals of Applied Biology 102: 331, 1983.
- Hernández, Visser, Brown & Bol, Journal of General Virology 78: 465, 1997.
- Horváth, Acta Phytopathologica Academiae Scientiarum Hungaricae 13: 51, 1978.
- Kurppa, Jones, Harrison & Bailiss, Annals of Applied Biology 98: 243, 1981.
- Legorburu, Robinson, Torrance & Duncan, Journal of General Virology 76: 1497, 1995.
- Legorburu, Robinson & Torrance, Journal of General Virology 77: 855, 1996.
- Lister, Virology 28: 350, 1966.
- Lister, Journal of General Virology 2: 43, 1968.
- Lister & Bracker, Virology 37: 262, 1969.
- Lister & Murant, Annals of Applied Biology 59: 49, 1967.
- Liu, Reavy, Swanson & MacFarlane, Virology 298: 232, 2002.
- Maat, Netherlands Journal of Plant Pathology 69: 287, 1963.
- MacFarlane, Journal of General Virology 80: 2799, 1999.
- Mayo & Cooper, Journal of General Virology 18: 281, 1973.
- Mayo, Brierley & Goodman, Biochimie 75: 639, 1993.
- Nixon & Harrison, Journal of General Microbiology 21: 582, 1959.
- Noordam, Tijdschrift over Plantenziekten 62: 219, 1956.
- Pelham, Virology 97: 256, 1979.
- Ploeg, Brown & Robinson, Netherlands Journal of Plant Pathology 98: 291, 1992a.
- Ploeg, Brown & Robinson, Annals of Applied Biology 121: 619, 1992b.
- Ploeg, Robinson & Brown, Journal of General Virology 74: 1463, 1993.
- Quanjer, Tijdschrift over Plantenziekten 49: 37, 1943.
- Roberts & Mayo, Journal of Ultrastructure Research 71: 49, 1980.
- Robinson, Journal of General Virology 64: 657, 1983.
- Robinson, Journal of Virological Methods 40: 57, 1992.
- Robinson & Harrison, Journal of General Virology 66: 171, 1985a.
- Robinson & Harrison, Journal of General Virology 66: 2003, 1985b.
- Robinson, Mayo, Fritsch, Jones & Raschké, Journal of General Virology 64: 1591, 1983.
- Robinson, Hamilton, Harrison & Baulcombe, Journal of General Virology 68: 2551, 1987.
- Rozendaal, Tijdschrift over Plantenziekten 53, 93, 1947.
- Sänger, Journal of Virology 3: 304, 1969.
- Sänger & Brandenburg, Naturwissenschaften 48: 391, 1961.
- Schmelzer, Phytopathologische Zeitschrift 28: 1, 1956.
- Schmelzer, Phytopathologische Zeitschrift 30: 281, 1957.
- Semancik, Phytopathology 56: 1190, 1966.
- Sudarshana & Berger Archives of Virology 143: 1535, 1998.
- Taylor & Brown, Nematode Vectors of Plant Viruses, Wallingford: CAB International, 1997.
- Taylor & Robertson, Journal of General Virology 6: 179, 1970.
- Uhde, Koenig & Lesemann, Archives of Virology 143: 1041, 1998.
- Uschdraweit & Valentin, Nachrichtenblatt des Deutschen Pflanzenschutzdienstes 8: 132, 1956.
- Van Belkum, Cornelissen, Linthorst, Bol, Pleij & Bosch, Nucleic Acids Research 15: 2837, 1987.
- Van Hoof, Netherlands Journal of Plant Pathology 76: 329, 1970.
- Van Hoof, Maat & Seinhorst, Netherlands Journal of Plant Pathology 72: 253, 1966.
- Van Slogteren, Tijdschrift over Plantenziekten 64: 452, 1958.
- Xenophontos, Robinson, Dale & Brown, Potato Research 41: 255, 1998.
- Zerfass & Beier, EMBO Journal 11: 4167, 1992.
- Ziegler-Graff, Guilford & Baulcombe, Virology 182: 145, 1991.