Details of DPV and References
DPV NO: 414 October 2006
Family: Potyviridae
Genus: Potyvirus
Species: Potato virus Y | Acronym: PVY
This is a revised version of DPV 242
Potato virus Y
Camille Kerlan INRA-Agrocampus Rennes, UMR BiO3P, Domaine de La Motte, F-35653 Le Rheu, France
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
PVY was first recognised in potato in 1931
(Smith, 1931)
as a member of a group of pathogens associated with potato degeneration, a disorder known since
the 18th century. It has been one of the most studied plant viruses.
It was the type species of the former Potyvirus group
(Harrison et al., 1971).
- Selected synonyms
- Potato acropetal virus
- Potato leafdrop streak virus
- Potato virus 20
- Solanum virus 2
- Tobacco veinal necrosis virus (quoted by de Bokx, 1987)
- Potato leafdrop streak virus
Brief description
PVY is the type species of the Potyvirus genus, one of the six genera in the
family Potyviridae
(Shukla et al., 1998).
PVY virions are filamentous, non-enveloped flexuous rods, 730-740 nm long, 11-12 nm in diameter,
with helical symmetry, containing about 6 % nucleic acid. The genome consists of one single-stranded
linear RNA molecule. PVY is naturally aphid-transmitted in the non-persistent manner,
Myzus persicae being the most efficient vector.
PVY is also sap-transmissible and graft-transmissible.
PVY has a large host range which includes not only cultivated solanaceous species but also many solanaceous and non-solanaceous weeds. It has a worldwide distribution and is one of the most economically important plant pathogens (Milne, 1988). It is responsible for severe diseases in widely cultivated crops (e.g. potato, tobacco, tomato, pepper) and ornamental plants (e.g. petunia). It displays great variability. Many strains, variants and pathotypes have been differentiated, the main strains being PVYO and PVYN.
Main Diseases
In potato, PVY causes a severe disease called mosaic or rugose mosaic.
Symptoms are variable depending on viral strain, host cultivar, climatic conditions, and whether it is
a primary infection (inoculation by aphid vectors) or secondary infection
(when mother tuber is infected)
(Draper et al., 2002).
Symptoms in the aerial parts of the plants consist of a mild to severe mottle
(Fig. 1;
Fig. 2),
often associated with distortion (crinkling) of the leaves.
Yellowing and necrosis (vein necrosis and necrotic spots) frequently occur in the lower leaves
(Fig. 3;
Fig. 4;
Fig. 5).
Symptoms also include collapse and dropping of intermediate leaves (leaf drop), which remain clinging
to the stem (Fig. 6).
Secondarily infected plants are dwarfed and brittle, with crinkled and puckered leaves
(Fig. 7).
Necrosis in tubers may occur in numerous cultivars
(Beemster & De Bokx, 1987).
The potato tuber necrotic ringspot disease
(Fig. 8;
Fig. 9;
Fig. 10;
Fig. 11;
Fig. 12),
described in the 1980's
(Beczner et al., 1984),
has now spread worldwide.
PVY usually causes mild mottling in tobacco, tomato and pepper. However a large range of various symptoms may be observed (Fig. 13; Fig. 14; Fig. 15) and many strains induce necrosis. The veinal necrosis disease in tobacco, formerly described as associated with tobacco veinal necrosis virus, is caused by the PVYN strain. Occurrence of necrotic symptoms in tobacco crops may be associated with unbalanced nutrition, notably magnesium deficiency (Delon et al., 1997). Necrotic patterns in tomato plants include veinal necroses and necrotic spots on leaves and sometimes necrotic streaks on petioles and stems (Gebre-Selassie et al., 1987). PVY in pepper is associated with mottle, vein banding and crinkling of the leaves and sometimes with severe leaf distortion and abscission, and stunting and collapse of the plants (reviewed by Edwardson & Christie, 1997). In eggplant (Solanum melongena), PVY (strain PVYC) induces a systemic mosaic with necrotic rings developing on lower leaves (Bala & Bhargava, 1977), stunting, and flower and fruit reduction (Edwardson & Christie, 1997). In petunia, PVY induces colour-breaking, leaf mosaic, yellow mottling, vein clearing and distortion of leaves (Fig. 16) (Boonham et al., 1999).
Surveys of the worldwide economic importance of PVY were reported by Tomlinson (1987), Milne (1988) and Shukla et al. (1994). PVY is in the top five viruses affecting field-grown vegetables (Milne, 1988). It was assessed to be the most important filamentous virus in Mediterranean countries (Martelli, 1988) and Southern Africa (Fauquet & von Wechmar, 1988), the second most important in Europe (Lecoq et al., 1988) and South America (Salazar & Mink, 1988), the fourth in China (Chiu, 1988), the sixth in Southeast Asia (Inouye et al., 1988), the seventh in the Indian subcontinent (Varma, 1988), and the tenth in North America (Mink, 1988).
In potato, PVY is currently the most economically important virus. PVYO, and to a lesser extent PVYN, are responsible for heavy losses both in secondary infections and in primarily-infected plants at an early stage. Losses up to 40-70% in the case of infections by PVYO have been recorded. Yield reduction is the result of fewer tubers being produced or a decrease in tuber size or both (Van der Zaag, 1987). In addition, tuber quality is severely affected in numerous cultivars susceptible to the potato tuber necrotic ringspot disease caused by the variant PVYNTN. Symptoms in foliage and growth reduction are more severe when PVY occurs in combination with other viruses, especially Potato virus X or Potato virus A.
PVY is also said to be the most damaging virus in tobacco crops (Blancard, 1998). A recent epidemic was reported in China (Li et al., 2001). Yield losses of 14-59 % were reported (Latore et al., 1982). PVY causes height reductions and modifies the chemical composition of cured leaves, especially the nicotine content (Latore & Flores, 1984). Losses can be up to 100% in the case of tobacco veinal necrosis disease.
PVY has been listed as one of the most economically important viruses in tomato and pepper in numerous countries in both outdoor and protected crops (Tomlinson, 1987). Necrotic strains cause serious yield and quality losses of tomato fruits in some cultivars (Gebre-Selassie et al., 1987; Legnani et al., 1995). PVY in pepper (numerous cultivars of Capsicum annuum and C. frutescens) is often associated with severe diseases (reviewed by Edwardson & Christie, 1997). Mixed infections with other pepper viruses such as Tobacco etch virus, Pepper mottle virus and Tobacco mosaic virus are frequent (Abdallah et al., 1991). PVY was reported in eggplant in India (Bhat et al., 1999), but seems to be rare in Europe (Moury, pers. comm.).
In the last decade, PVY has also been found to be responsible for severe damage in trailing petunias, which are now vegetatively propagated (Bellardi et al., 1996).
Geographical Distribution
PVY is distributed worldwide in potato growing areas, in tobacco growing areas and in outdoor crops of tomato and pepper in warm countries. In potato crops, the PVYO strain occurs worldwide (Jeffries, 1998). The PVYN strain has been recorded in South America, Europe, Africa, Asia (Weidemann, 1988a) and New Zealand (Fletcher, 1989), whereas it is a quarantine pathogen in Canada (Ellis et al., 1997) and USA, with localised outbreaks (Singh et al., 1993; Croslin et al., 2002). The PVYC strain, known in Europe, North America, India, South Africa, Australia, New Zealand and Ecuador (Ellis et al., 1997; Jeffries, 1998), is probably more prevalent than commonly accepted. The PVYNTN variant has been identified in most potato-growing countries around the world, including USA (McDonald & Singh, 1996b; Croslin et al., 2002; Nie & Singh, 2003b), Japan (Ohshima et al., 2000) and Peru (Salazar et al., 2000). The PVYN-W variant has become prevalent in Poland (Chrzanowska, pers. comm.) and has been reported in a few other countries including France, Spain (Blanco-Urgoiti et al., 1998a; Kerlan et al., 1999a), Germany and Russia (Chrzanowska, pers. comm.). PVYN:O was reported in Canada (Manitoba) and USA (Minnesota, Montana, North Dakota) (Singh et al., 2003).
Host Range and Symptomatology
The natural host range is wide and comprises up to nine families including important crops such as
pepper (Capsicum spp.), potato (Solanum tuberosum ssp. tuberosum),
tobacco (Nicotiana spp.), tomato (Lycopersicon esculentum), some ornamental plants
(Dahlia and Petunia spp.) and many weeds (Datura spp., Physalis spp.,
Solanum dulcamara and S. nigrum)
(Jeffries, 1998).
New naturally infected host species were recorded in New Zealand: Cotula australis and
Capsella bursa-pastoris
(Fletcher, 2001).
The experimental host range is reported to comprise 495 species in 72 genera of 31 families including 287 species in 14 genera of the Solanaceae (among which 141 Solanum species and 70 Nicotiana species), 28 species of Amaranthaceae, 25 species of Leguminosae, 20 species of Chenopodiaceae, and 11 species of Compositae (compiled by Edwardson & Christie, 1997).
- Diagnostic species:
- Species susceptible to all PVY strains include:
Nicotiana tabacum: interveinal clearing 10-14 days p.i. followed by mottle, and depending on the strain, leaf distortion, growth reduction and/or necrotic patterns (Fig. 17; Fig. 18).
N. benthamiana: systemic vein clearing, mottle and rugosity (Jeffries, 1998).
N. occidentalis-P1: sometimes mild chlorotic local lesions, systemic vein clearing, mottle, chlorosis and stunting (Jeffries, 1998).
Datura stramonium is stated to be immune to all tested strains (De Bokx & Huttinga, 1981; Le Romancer et al., 1994), though reported as a species infectible by PVY (Bawden & Kassanis, 1947).
- Species that are useful for distinguishing among PVY strains and pathotypes include:
N. tabacum: cvs White Burley, Samsun NN and Xanthi are useful for differentiating strains of the PVYN group from strains of the other groups. PVYN induces in all these cvs a severe veinal necrosis, associated with a systemic vein banding in cv White Burley, with smaller distorted leaves and dwarfing of the whole plant in cv Xanthi (Fig. 18), and with some isolates collapse and death of the plant. PVYO and PVYC induce only vein banding or interveinal clearing (Fig. 17) followed by a mottling which may later decline and disappear. Cvs and genotypes Mac Nair 944, NC 95, Burley 21, VD, Virginia A mutant and NC TG52 are used to differentiate PVY tobacco pathotypes (Gooding, 1985; Blancard et al., 1995).
Physalis floridana: PVYO and PVYC cause local and systemic necrosis in young plants. Collapse and death of the whole plant follow inoculation with PVYC. PVYN induces mosaic symptoms only.
S. tuberosum ssp. tuberosum (potato): cvs Desiree, Eersteling and Maris Bard react hypersensitively after mechanical inoculation with or grafting to stocks infected by PVYO, PVYC and PVYZ, respectively (Fig. 19; Fig. 20; Fig. 21), whereas typical PVYN isolates do not induce local necrotic lesions in inoculated leaves (Jones, 1990; Kerlan et al., 1999a). Cvs Nicola, Nadine and Hermes display tuber necrosis when infected by PVYNTN (Fig. 22) (Browning et al., 2004). Potato cultivars resistant to Potato virus X, such as Saco, can be used to separate PVY from PVX in mixed infections whereas D. stramonium is used to eliminate PVY from mixed infections.
S. brachycarpum and S. sparsipilum: some accessions can be used for specific identification of PVYN (Singh et al., 1994).
Chenopodium amaranticolor: PVYO and PVYC cause local lesions 10-14 days p.i. (Fig. 23) whereas PVYN does not.
Capsicum annuum: cvs Bastidon, Yolo Y and Florida VR-2, are pepper cultivars that differentiate three pathotypes of pepper isolates of PVY (Gebre-Selassie et al., 1985).
- Propagation species:
Nicotiana tabacum cv Xanthi (Gugerli & Fries, 1983; Sanz et al., 1990), cv White Burley (Rose & Hubbard, 1986), or cv Samsun (Ellis et al., 1996) are good host plants for virus purification.
- Assay species:
Solanum demissum: S. demissum Y and the clone S. demissum A6 (S. demissum × S. tuberosum cv Aquila) are local lesion hosts including for PVYN (De Bokx, 1987; Beemster & De Bokx, 1987). The "A6 test" was commonly used in the past as a diagnostic tool for differentiating PVY from Potato virus A (PVA). Local lesions are visible 2 days p.i in detached leaves (De Bokx, 1987). S. demissum PI 230579 is stated to be a better local lesion host than A6 (Webb & Wilson, 1978). S. demissum A gives local lesions after infection with PVA, but not with PVY (De Bokx, 1987).
N. tabacum plantlets are often used as source and test plants for aphid transmission experiments.
Chenopodium amaranticolor was also used as test plant in aphid transmission experiments (Makkouk & Gumpf, 1976).
Strains
Potato isolates have historically been divided into three main strain groups: PVYO, PVYN and PVYC according to symptoms induced in N. tabacum cv Samsun, S. tuberosum ssp. tuberosum and Physalis floridana (De Bokx & Huttinga, 1981). PVYO and PVYC are separated on the basis of hypersensitive reactions in potato cultivars bearing different resistance genes, namely Nytbr and Nc for PVYO and PVYC, respectively (Cockerham, 1970; Jones, 1990). PVYN differs from PVYO and PVYC in causing a severe veinal necrosis reaction in tobacco, but elicits a hypersensitive response in few if any potato cultivars (Jones, 1990; Valkonen, 1997). Some (but not all) PVYC isolates are not aphid transmissible (Watson, 1956; De Bokx & Piron, 1978; Blanco-Urgoiti et al., 1998b). The virus formerly called Potato virus C (PVC) (non-aphid transmissible) has been demonstrated to be a PVY strain (Cockerham, 1943; Bawden & Kassanis, 1947), and is included in PVYC.
In the past, a fourth group called PVYAn (Horvath, 1967b) was described which included particular potato and tomato isolates. Many other variants have been reported, including: isolates that do not induce necrosis in S. demissum A6 (De Bokx et al., 1975; Thompson et al., 1987); PVYZ and PVYZE, which are particular pathotypes found in Britain, Spain and France that overcome the Nytbr and Nc genes (Jones, 1990; Blanco-Urgoiti et al., 1998a; Kerlan et al., 1999a); PVYNTN, which is characterised by its necrotic properties in potato tubers (Le Romancer et al., 1994); PVYN-W (Chrzanowska, 1994; Chachulska et al., 1997; Glais et al., 1998) and PVYN-O (Singh et al., 2003), which share properties with both PVYN and PVYO. Several non-typical isolates were mistakenly considered to belong to the PVYC strain group (Rozendaal et al., 1971; De Bokx et al., 1975; Calvert et al., 1980) and were later recognised as isolates of the newly described Potato virus V (Jones & Fribourg, 1986).
PVY tobacco isolates are broadly defined as "severe" or "mild", depending whether or not they induce necrosis in any tobacco genotype (Gooding, 1985). Historically, three strain groups, MSMR, MSNR, NSNR, have also been identified according to their reaction in tobacco cultivars resistant or susceptible to the root-knot nematode (Meloidogyne incognita) (Gooding & Tolin, 1973). A resistance-breaking group of isolates designated VAM-B was also detected in tobacco crops (Latore & Flores, 1985; Gooding, 1985). Blancard et al. (1995) proposed a new nomenclature differentiating six pathotypes according to their behaviour in four tobacco genotypes.
Tomato isolates of PVY have been separated into necrotic isolates (inducing mosaic and necrosis) and non-necrotic isolates (causing only mosaic) (Marchoux et al., 1995).
PVY isolates infecting pepper have been classified into three pathotypes designated PVY-0, PVY-1 and PVY-1-2 according to their ability to overcome resistance genes (vy1, vy2) present in pepper cultivars Bastidon, Yolo Y and Florida VR-2 (Gebre-Selassie et al., 1985). Within these three groups, pepper isolates have been further defined as "common" or "necrotic" (d'Aquino et al., 1995).
Some studies demonstrated a high level of host specificity (Gebre-Selassie et al., 1985; McDonald & Kristjanson, 1993; d'Aquino et al., 1995). In contrast, several isolates from potato and tobacco were shown to infect cultivars of Capsicum annuum (Horvath, 1966a, 1966b, 1967a; Marte et al., 1991; McDonald & Kristjanson, 1993; Le Romancer et al., 1994) and of tomato (Stobbs et al., 1994; Legnani, 1995).
Molecular classifications based on genome polymorphism, either considering the whole RNA or more frequently a single region, distributed PVY isolates into two or three main clusters (Van der Vlugt et al., 1993; Marie-Jeanne Tordo et al., 1995). Three genetically defined strains were established on the basis of RFLP typing of the coat protein gene: PVYO, PVYN and PVYNP (non-potato), this last including isolates from pepper, tobacco and Datura species (Blanco-Urgoiti et al., 1996). By using the same typing, PVYC isolates were recently characterised as a homogeneous pathotype but divided into two genetically distinct strains designated PVYC1 and PVYC2, PVYC1 isolates being included in the PVYNP cluster whereas PVYC2 was found to be separate from the other PVY groups or subgroups described so far (Blanco-Urgoiti et al., 1998b). Pepper isolates were shown to form a single genetic strain separated from other PVY strains (Llave et al., 1999a; Romero et al., 2001).
PVYNW, PVYN:O and the majority of PVYNTN isolates are recombinants whose genome displays PVYO-type segments and PVYN-type segments (Fig. 24) (Revers et al., 1996; McDonald & Singh, 1997; Glais et al., 1998; Boonham et al., 1999; Singh et al., 2003). PVYNW and PVYN:O isolates induce a vein necrosis reaction in tobacco and possess a PVYO-type coat protein (McDonald & Singh, 1996a; Chachulska et al., 1997; Glais et al., 1998; Nie & Singh, 2003a). All PVYNW isolates tested were shown to display one or two recombination breakpoints (Glais et al., 2002a). Most PVYNTN isolates (i.e. inducing potato tuber necrosis under natural conditions) display recombination breakpoints in the coat protein gene and two other genomic regions (HC-Pro/P3 and CI/6K2) (Revers et al., 1996; Glais et al., 1998; Boonham et al., 1999). However many tuber necrosis-inducing isolates, including one North-American PVYNTN isolate (Nie & Singh, 2003b), possess a PVYN-type genome without any recombination breakpoint (Ohshima et al., 2000; Glais et al., 2001; Boonham et al., 2002b). North-American (NA)- and European (EU)-PVYNTN isolates were separated on the basis of phylogeny using the P1 and 5' non-translated regions (Nie & Singh, 2002a).
Transmission by Vectors
PVY is an unusual potyvirus in having numerous vector aphid species
(Shukla et al., 1994).
Compilations of aphid species reported to transmit PVY were provided by
Kennedy et al. (1962),
Peters (1987a), and
Edwardson & Christie (1997).
26 species or species groups, all in the family Aphidinae, were identified as vectors of PVY
by Harrington & Gibson (1989).
All except Uroleucon sp. transmitted PVYO. Nine species or species groups
transmitted PVYN: Acyrthosiphon pisum
(Fig. 25),
Aphis fabae, Aphis nasturtii, other Aphis spp., Brachycaudus helichrysi,
Myzus cerasi, M. persicae
(Fig. 26),
Phorodon humuli and Uroleucon sp. Five of the 26 species mentioned as PVY vectors by
Harrington & Gibson (1989)
were found not to be vectors of PVYN by
De Bokx & Piron (1990):
Aphis pomi, Cryptomyzus ballotae, Myzaphis rosarum, Myzus myosotidis,
and Metopolophium festucae, whereas 6 other species not recorded by
Harrington & Gibson (1989)
were found to transmit PVYN by
De Bokx & Piron (1990):
Brachycaudus spp., Capitophorus hippophaes, Cryptomyzus galeopsidis,
Hyadaphis foeniculi, Hyalopterus pruni, and Myzus certus.
Edwardson & Christie (1997)
listed 41 aphid species reported to transmit PVY. This list omitted Cavariella aegopodii which
was found to be a poor vector of PVY
(Piron, 1986;
Harrington & Gibson, 1989;
De Bokx & Piron, 1990)
and Drepanosiphum platanoïdis which was found to be a possible very poor vector by
Powell et al. (1992).
In potato crops in most areas and seasons, M. persicae (Fig. 26) is clearly the most important vector given that it is widespread and that its efficiency of transmission is high. Efficiency of transmission by other vector species is comparatively low or very low (Kostiw, 1975; Van Harten, 1983; Sigvald, 1984), but despite this low efficiency, some species are noteworthy vectors of PVY, either species that colonise potatoes such as A. nasturtii (in Central and Eastern Europe), Macrosiphum euphorbiae (Fig. 27) and Aulacorthum solani (De Bokx, 1987; Peters, 1987b), or species that visit but rarely colonise potatoes (Ryden et al., 1983). 22 such visiting species were quoted by Peters (1987a), and some of them (R. padi, A. pisum, B. helichrysi, Metopolophium dirhodum, C. aegopodii) are possibly involved in epidemics due to PVY (Kerlan et al., 1987; Weidemann, 1988a). Studies on the relative importance of different aphid species as PVY vectors in Southern England proved that B. helichrysi and M. persicae accounted for about half of all observed transmissions, but that Phorodon humili and many Aphis species also played a significant role (Harrington et al., 1986; Harrington & Gibson, 1989).
PVY is transmitted in a non-persistent manner which means brief acquisition and inoculation periods (a few seconds or minutes for acquisition, a few seconds for inoculation). There is no discernible latent period. Acquisition and inoculation involve stylet penetrations into the epidermal cell layer of the plants and occur when stylets puncture plant cell membranes, though the possibility for the virus to be acquired and inoculated via broken plasmodesmata is not totally excluded (Powell, 1991; Lopez-Abella et al., 1988). Retention of the virus in aphids in most cases lasts not more than one or two hours (Proeseler & Weidling, 1975; Peters, 1987a). However, longer retention periods were reported, with significant differences according to the viral strain and the aphid vector species: up to 4 hours and 8 hours in Myzus persicae and Phorodon humuli, respectively (Van Hoof, 1980) and more than 17 hours in winged forms of Aphis nasturtii (Kostiw, 1975). Such a long retention period may explain why PVYN isolates can be transmitted over rather long distances (Van Hoof, 1977, 1979). The virus does not pass through the moult.
Prior starvation of the aphids increases the efficiency of transmission (Watson & Roberts, 1939) though it does not affect the occurrence of electrically-recorded membrane punctures during acquisition access (Powell, 1993). Starving periods of 1-4 hours were reported (Kostiw, 1975; Powell et al., 1995). The efficiency of transmission depends on many other factors including the nature of the source and test plants (Van Hoof, 1980; Katis & Gibson, 1985), the virus concentration in the source plant (De Bokx et al., 1978), the mature-plant resistance in potato (Sigvald, 1985; Beemster, 1987; Gibson, 1991), the environmental conditions and the viral strain, PVYN isolates being better transmitted than other PVY isolates (Proeseler & Weidling, 1975). Interference between PVY strains during aphid transmission has been reported: transmission of PVYO decreased when aphids had previously or subsequently fed on PVYN-infected source plants (Katis et al., 1986).
PVY transmissibility is determined by two viral gene products: the helper component protein (HC) and the coat protein (CP) (Pirone, 1991; Pirone & Blanc, 1996). It was first proved that a virus-induced component of the plant sap is needed for aphid transmission of PVY (Govier & Kassanis, 1974a, 1974b). HC of an efficiently transmitted PVY isolate can mediate the transmission of non- or poorly aphid-transmitted PVY isolates and of other Potyviruses such as Potato aucuba mosaic virus, Tobacco etch virus, Henbane mosaic virus and Plum pox virus (Kassanis & Govier, 1971; Govier & Kassanis, 1974b; Pirone, 1981; Lopez-Moya et al., 1995). PVY HC was purified (Govier et al., 1977; Pirone & Thornbury, 1983; Thornbury et al., 1985; Powell et al., 1995) and determined by SDS-PAGE to have a subunit molecular mass of 58 kDa, whereas the biologically active form (apparently dimeric) had a molecular mass of 100-150 kDa (Thornbury et al., 1985). Polyclonal antisera and monoclonal antibodies to PVY HC were prepared (Hiebert et al., 1984a; Thornbury et al., 1985; Canto et al., 1995a). The HCs of PVY and of Tobacco vein mottling virus appeared to be serologically different (Thornbury & Pirone, 1983). Antisera to PVY HC were found to block the biological activity of the HC of PVY, but not the activity of HCs of other potyviruses (Thornbury et al., 1985; Dougherty & Carrington, 1988). HCs of aphid-transmissible and non-aphid-transmissible PVY isolates were used to demonstrate that loss of potyvirus transmissibility and helper-component activity correlate with non-retention of virions on aphid stylets (Wang et al., 1996).
Sequence comparisons of HCs of aphid-transmissible (PVYN) and non-transmissible (PVC) isolates revealed two amino acid substitutions specific to the non-transmissible isolate: Lys to Glu at position 50 in the N-terminal cysteine-rich, metal-binding region, and Ile to Val at position 225 in the middle of the HC sequence (Thornbury et al., 1990). The first of these is part of a conserved KITC motif in potyvirus HCs and all changes in or around this motif have led to decreased stability of HC, modifying its dimeric form, and have resulted in losses of aphid transmissibility (Canto et al., 1995b; Maia et al., 1996; Legavre et al., 1996). The Lys to Glu mutation in PVC does not hinder binding of HC to capsid protein but renders the HC incapable of interacting with aphid stylets (Blanc et al., 1998). Biologically active forms of HCs of two PVY strains have been cloned, expressed from a Potato virus X vector and purified by metal affinity chromatography. This system was used to confirm that reversal of the Lys to Glu mutation restores the helper function of defective PVC HC (Sassaya et al., 2000). Other single amino acid substitutions reported to abolish the biological activity of HC in many pepper isolates include Gly to Asp in the cysteine-rich region, Ser to Gly in the carboxy-terminal region (Canto et al., 1995b), Arg to Gln at position 89 and Met to Ile at position 152 (Llave et al., 1999b).
The N-terminal domain of PVY CP is also involved in aphid transmissibility. This domain contains the DAG triplet found in all PVY aphid transmissible isolates examined (Shukla et al., 1991), and also in the non-aphid transmissible isolate PVY-18 (Shukla et al., 1988c). Unlike Tobacco vein mottling virus, PVY isolates having the sequence DAGE are aphid-transmissible (Shukla et al., 1994).
Transmission by non-aphid vectors. PVY transmission by the red spider mite (Tetranychus urticae) (Schulz, 1963) was not confirmed (Fritzsche et al., 1967; Orlob, 1968).
Transmission by contact. It has been stated that PVY may be spread mechanically between potato plants by plant-to-plant contact (Banttari et al., 1993), and some PVYN isolates are said to be spread by plant-to-plant contact in tobacco and tomato (C. Jeffries, pers. comm.). In potato, damage to the sprouts or skin of the tubers seems enough to create an entry site for the virus. Indeed by handling sprouted tubers some of which carried PVYN, e.g. when removing sprouts by hand or when tubers are put through brushing machines, PVY was transmitted to 30% of the healthy tubers (De Bokx, 1987).
Transmission through Seed
PVY is not mentioned in the list of potato viruses transmitted through true seed or pollen of potatoes, though complete absence of seed transmission is difficult to demonstrate with certainty (De Bokx, 1987).
Transmission by Dodder
PVY is able to infect Cuscuta gronovii (Edwardson & Christie 1997), but no further information is available.
Serology
PVY is strongly immunogenic (Shukla et al., 1994). Antisera with precipitin titres of 1/4096 have been readily obtained (Maat & Huttinga, 1987). Monoclonal antibodies have been produced in mice immunised with purified virus preparations (Gugerli & Fries, 1983; Rose et al., 1987; Ohshima et al., 1990; Sanz et al., 1990; Singh et al., 1993; Ellis et al., 1996; Cerovská, 1998). Antisera and monoclonal antibodies have also been produced against synthetic peptides (Ohshima et al., 1992; Vuento et al., 1993; Liu et al., 1999; Ounouna et al., 2002). Recombinant antibodies (single chain Fv antibody fragments) have been obtained by using phage display antibody technology (Boonham & Barker, 1998). Antibodies have also been generated by DNA-based immunization of rabbits (Hinrichs et al., 1997).
ELISA is in current usage to detect the virus from leaves or other organs such as potato tubers according to the basic double antibody sandwich (DAS) protocol (Marco & Cohen, 1979; Boonham et al., 2002b). Antigen coated plate-ELISA (Singh & Barker, 1991; Barker et al., 1993) and triple antibody sandwich-ELISA are also frequently used. ELISA testing has been found to be unsatisfactory on dormant tubers (De Bokx et al., 1981; De Bokx & Cuperus, 1987) and more reliable when tuber dormancy is broken by Rindite treatment (Gugerli & Gehriger, 1980; Vetten et al., 1983). DAS-ELISA has been used for PVY detection in the aphid M. persicae (Carlebach et al., 1982). Dot-ELISA or dot blot immunobinding assay (DBIA) on nitrocellulose membranes was said to have some advantages over the standard ELISA (Banttari & Goodwin, 1985; Berger et al., 1985; Weidemann, 1988b). Immunosorbent electron microscopy (ISEM) was optimized by Cohen et al. (1982), who studied the effect of different buffers for extraction of the virus from tobacco, tomato and Physalis floridana. ISEM was used for studying antigenic relationships between different PVY strains (Gebre-Selassie et al., 1985) and different potyviruses (Walkey & Webb, 1984). Immunodiffusion tests (Purcifull & Gooding, 1970) can be done in agar gels using virus degraded by dissociating agents such as sodium dodecyl sulphate (Makkouk & Gumpf, 1976; Purcifull & Batchelor, 1977). The latex agglutination test (Gnutova & Krylov, 1975a; Talley et al., 1980; Torrance, 1980), simpler but much less sensitive than ELISA, has been recommended by the International Potato Centre for routine diagnosis in developing countries (L. Salazar, pers. comm.). A variation of this test, the virobacterial agglutination test, in which antibodies are coated onto bacterial cells (Chirkov et al., 1984), was described as a specific and rather sensitive technique for PVY detection (Walkey et al., 1992). Microprecipitin tests, used up to the 1980's, were of moderate sensitivity and reliability.
Antibodies are available in type-culture collections (e.g. the American Type Culture Collection). ELISA kits are available from commercial companies.
Nucleic Acid Hybridization
Relationships
Polyclonal antisera do not discriminate different PVY strains (Heath et al., 1987; Rose et al., 1987; Ellis et al., 1996). Monoclonal antibodies (MAbs) detecting a broad spectrum of PVY isolates (Fernandez-Northcote & Gugerli, 1987) have been produced. MAbs specific to or detecting most isolates of PVYN, PVYO or PVYC, with no or little cross-reaction to other strains, have been reported (Gugerli & Fries, 1983; Ellis et al., 1996; McDonald & Singh, 1996a; Ounouna et al., 2002). However the reliability of some commercial ELISA kits has still to be proved, especially for detecting specifically PVYC. MAbs to the PVYNTN strain have been reported (Čeŕovská, 1998) as well as a MAb reacting specifically with PVYN-W-like isolates (McDonald et al., 1997b), but a greater range of isolates requires to be tested to determine the reliability of these reagents. PVYO specific recombinant antibodies have been obtained by Boonham & Barker (1998).
Contradictory results in cross-protection tests between PVY strains have been reported; either antagonistic effects (Aubert, 1958; Richardson, 1958; Todd, 1961; Ramirez et al., 1964; De Bokx et al., 1975; Makkouk & Gumpf; 1976, Gooding, 1985), or lack of cross-protection (Bawden & Kassanis, 1951; Schmelzer et al., 1960; Beczner et al., 1984; Le Romancer et al., 1994). Recent molecular studies on recombinant variants (Glais et al., 2002b) tend to show that PVYO and PVYN strains can co-exist in the same plant cells.
Nucleotide sequence identities among PVY isolates range from 93 to 99% in the coding region, irrespective of the gene product being considered, and from 83 to 98% in the 3' non-coding region (Van der Vlugt et al., 1993; Shukla et al., 1994). Overall identities of nucleotides are 97% or higher among PVYO isolates (Nie & Singh, 2002a) and about 92% to 99% among PVYN isolates (Nie & Singh, 2003a). The identity between PVYO and PVYN groups falls to 72% in the least conserved region, the P1 gene (Marie-Jeanne Tordo et al., 1995; Singh & Singh, 1996) and reachs 95% in the most conserved region, the CI gene (Nie & Singh, 2003a). Comparisons of polyprotein sequences between PVY and other potyviruses show high variation in P1, P3 and the N-terminal part of the CP, whereas some domains of HC, CI and NIb are highly conserved (Shukla et al., 1991).
PVY is distantly serologically related to two other potato potyviruses, Potato virus A (PVA) and Potato virus V (PVV) (Fribourg & Nakashima, 1984), and to other distinct potyviruses such as: Bean common mosaic virus, Bidens mottle virus, Beet mosaic virus, Bean yellow mosaic virus, Celery mosaic virus, Henbane mosaic virus, Lettuce mosaic virus, Maize dwarf mosaic virus, Plum pox virus, Papaya ringspot virus, Pepper veinal mottle virus, Passion fruit woodiness virus, Pokeweed mosaic virus, Tobacco etch virus (TEV), Turnip mosaic virus and Watermelon mosaic virus (Bercks, 1960; Bartels, 1964; Shepherd et al., 1969, Shepard et al., 1974; Brunt et al., 1978; De Bokx & Huttinga, 1981; Edwardson & Christie, 1997). PVY is also distantly related to several but not all isolates of Pepper mottle virus (PepMoV) (Purcifull et al., 1973, 1975a; Nelson & Wheeler, 1978; Shukla et al., 1994). PepMoV, first described as an atypical isolate of PVY (Zitter, 1972), has been shown to be a distinct species since the two viruses differ from each other in many properties (Hiebert & Purcifull, 1992), including host range, lack of cross-protection, type of cytoplasmic inclusions and genomic sequences (Vance et al., 1992; Shukla et al., 1994). Most of the serological relationships with other potyviruses are due to common epitopes located in the conserved core region of the PVY coat protein (Shepard et al., 1974; Jordan & Hammond, 1991; Shukla et al., 1992), the relationship to TEV being an exception (Shukla et al., 1989). Phylogenetic comparisons indicated that PVY, PepMoV, PVV, Pepper yellow mosaic virus, Pepper severe mosaic virus, Wild potato mosaic virus and Peru tomato virus constitute a group distinguishable from other potyviruses including PVA (Spetz et al., 2003).
Stability in Sap
Thermal inactivation point (10 min) in tobacco sap has been estimated to vary from 50°C for an isolate from tomato (Clark & Hill, 1978) to 74°C for a PVYAn isolate (Horvath, 1967b). It was also recorded to be 56-72°C for PVYO, 64°C for PVYN, and 58-60°C for PVYC (Horvath, 1966a, 1966b, 1967a).
Dilution end-point is 1 × 10-4 to 2 ×10-6 for PVYO, 2 ×10-6 for PVYN, and 2 ×10-1 to 1 ×10-4 for PVYC (Horvath, 1966a, 1966b, 1967a).
Longevity in vitro (18-22°C) was recorded from one day (Clark & Hill, 1978) to 50 days (Klinkowski & Schmelzer, 1957). It was reported to be 18-31 days for PVYO, 21-27 days for PVYN, and 15-18 days for PVYC (Horvath, 1966a, 1966b, 1967a).
Infectivity in sap from tobacco leaf tissue containing 1% sodium azide is preserved at 25°C for 4 weeks (Gooding & Tsakiridis, 1971), and is not changed by treatment with diethyl ether.
Leaves are the best virus-infected material to store. PVY in potato or tobacco foliage can be stored effectively at -18°C. For long-term preservation, PVY-infected samples, dried and stored over calcium chloride at 4°C, can remain infective for 15 years (De Bokx, 1987), though inoculation from such material may sometimes be unsuccessful. PVY can also be preserved long-term by storing clarified virus-containing sap in or over liquid nitrogen (De Wijs & Suda-Bachman, 1979) or by lyophilization (Van Regenmortel, 1982). Antigenic properties can be retained for one year in freeze-dried crude extracts from infected N. tabacum plants (Purcifull et al., 1975b).
Purification
Most purification methods currently used are improvements of formerly described methods: Delgado-Sanchez & Grogan (1966), Darmidagh & Shepherd (1970), Huttinga (1973), Shepard et al. (1974), Gnutova & Krylov (1975b), McDonald et al. (1976) modified by Rose & Hubbard (1986), Moghal & Francki (1976) modified by Sanz et al. (1990), Leiser & Richter (1978), Gugerli (1978), Gugerli (1984), Hammond & Lawson (1988) modified by Chandelier et al. (2001). Na2-EDTA, urea, citrate and Triton X-100 have been used to prevent aggregation of the particles. Clarification has been done with chloroform, diethyl ether or carbon tetrachloride, singly or in combination. Further purification has been achieved by ammonium sulphate precipitation, ultracentrifugation through a layer of sucrose, and caesium chloride or sucrose gradient centrifugation (Huttinga & Maat, 1987). Yields in mg/kg of infected leaves varied from 9 to 23 (Makkouk & Gumpf, 1976) and 46 to 116 (Hammond & Lawson, 1988).
Good results can be obtained using the method reported by Leiser & Richter (1978) with a final centrifugation stage in caesium chloride (Kerlan, unpublished). Homogenize 100 g leaf tissue (N. tabacum cv Xanthi) in 300ml 0.5 M citrate buffer, pH 7.4, containing 5 mM Na2-EDTA and 15 mM sodium DIECA. Filter the homogenate through muslin and centrifuge for 15 min at 4360 g (6000 rpm). Add Triton X-100 to a final concentration of 3% (v/v) and stir for 30 min in a cold room. Centrifuge for 2 hours at 31 000 g. Resuspend the pellets in 10 mM citrate buffer, pH 7.4, containing 1M urea and 0.1 % 2-mercaptoethanol. After keeping overnight at 4-6°C, centrifuge for 15 min at 4360 g. Layer the supernatant fluid over a cushion of 20% (w/v) sucrose and centrifuge for 2 h at 50,000 g. Resuspend the pellets in 5mM sodium borate buffer, pH 8. Layer aliquots (0.3 ml/tube) over a CsCl gradient (0.47 g/ml) in 5mM sodium borate buffer, pH 8, and centrifuge for 5 h at 110,000 g (40,000 rpm in Beckman rotor 70.1 Ti) at 12°C. Recover the virus band identified by spectrophotometric analysis of the gradient fractions at 254 nm, dialyze against 5mM sodium borate buffer, pH 8, overnight at 4-6°C and centrifuge for 3 hours at 146,000 g (40,000 rpm) at 4°C.
Properties of Particles
Sedimentation coefficient: 138 - 148S depending on molarity and content of the phosphate buffer (Stace-Smith & Tremaine, 1970); 145S at infinite dilution in 0.1 M Tris-HCl, pH 9, at 20°C (Huttinga, 1975); 154S (Delgado-Sanchez & Grogan, 1966; Moghal & Francki, 1976); 151S in 0.01 M orthoborate pH 8.2 (Makkouk & Gumpf, 1976).
Buoyant density in CsCl : 1.323 gm.cm-3 for PVYO and 1.326 gm.cm-3 for PVYN (Huttinga, 1975).
Buoyant density in a non-ionic medium : 1.23 gm.cm-3 (Gugerli, 1984).
Absorbance at 260 nm, 1 mg. ml-1, 1 cm light path: 2.8-2.9, not corrected for light scattering (Stace-Smith & Tremaine, 1970; Leiser & Richter, 1978); 2.3, corrected for light scattering (Leiser & Richter, 1978).
A260/A280 corrected for light-scattering : 1.21 ± 0.04 (Stace-Smith & Tremaine, 1970); 1.21-1.22 (Leiser & Richter, 1978).
Particle Structure
PVY virions are filamentous, flexuous rods (Fig. 28) with an obscure axial canal 2-3 nm in diameter, a modal length of 740 nm and a width of 11 nm (Shukla et al., 1994). Measurements of length of PVY particles compiled by Edwardson & Christie (1997) ranged from 650 nm (Procenko, 1970) to 776 nm (Skofenko et al., 1975).
Optical diffraction patterns show the virions to have a helical structure with the mean pitch of the helix being 3.3 nm (Varma et al., 1968; Goodman et al., 1976). The estimated number of protein subunits per turn of helix is 7.7 (Veerisetty, 1978, quoted in Tollin & Wilson, 1988). The estimated number of nucleotides associated with each protein subunit is six (Veerisetty, 1979).
Assembly of protein subunits of PVY into intact but non-infective particles, and disruption of PVY particles were studied in detail by McDonald et al. (1976), Goodman et al. (1976) and McDonald & Bancroft (1977). Schematic drawings showing the assembly of the PVY particle, the sub-unit folding pattern and the tertiary structure of the PVY particle protein were presented by Shukla & Ward (1989) and Shukla et al. (1994). The predicted secondary structure of the PVY coat protein indicates four major antiparallel segments (Shukla et al., 1988b) with four extensive alpha-helical sections (at residues 62-80, 87-100, 145-174, and 209-229 in isolate PVY-D) and four beta-sheet sections (Shukla & Ward, 1989; Shukla et al., 1994).
Particle Composition
Nucleic acid: The nucleic acid of PVY is a single-stranded linear RNA with a sedimentation coefficient of 25S (Makkouk & Gumpf, 1974) to 39S (Makkouk & Gumpf, 1975), and a molecular weight of 3.1 x 106 (Makkouk & Gumpf, 1974) to 3.2 x 106 (Hinostroza-Orihuela, 1975). It has a VPg at the 5' terminus and a polyA sequence at the 3' terminus. No subgenomic RNA is produced. The percentage of RNA in particles is from 5.4 to 6.4% (Stace-Smith & Tremaine, 1970; Makkouk & Gumpf, 1974; Leiser & Richter, 1978).
Protein: Protein content in the particle is about 94%. Only two proteins, VPg and coat protein (CP), are detected in viral particles. CP molecular weight was calculated to be 29.95 kDa (Shukla et al., 1986). Other previously reported values (Miki & Oshima, 1972; Hiebert & McDonald, 1973) were either underestimated (Shukla et al., 1994) or resulted from probable proteolysis by host plant enzymes (Huttinga & Mosch, 1974; Moghal & Francki, 1976). Amino-acid composition has been determined by Stace-Smith & Tremaine (1970), Miki & Oshima (1972), Makkouk & Gumpf (1975), and Moghal & Francki (1976).
The coat protein consists of 267 amino acid residues, except for that of isolate PVY 18, which has a deletion at position 25 (Shukla et al., 1988c). Complete and partial amino acid sequences of the CP have been obtained (Shukla et al., 1986, 1988a, 1988c, 1994; McKern et al., 1992). Around 200 CP sequences are available in databases. N-terminal residues of the CP were not blocked in any of the five PVY isolates studied (Shukla et al., 1986, 1988c).
No lipid or other components have been detected in the particles.
Genome Properties
Complete genome sequences have been published or are available in sequence databases for four isolates of PVYN (Robaglia et al., 1989, accession number X12456 = NC_001616; Jakab et al., 1997, X97895; Abdelmaksoud & Gamal Eldin, unpublished, AF522296; Nie & Singh, 2003b, AY166867), two of PVYNTN (Thole et al., 1993, M95491; Nie & Singh, 2003b, AY166866), one of PVYO (Singh & Singh, 1996, U09509), and isolates from pepper (Crescenzi et al., unpublished, AF237963), tomato and Solanum nigrum (Moury et al., 2002, AJ439545and AJ439544, respectively). The complete sequence of the polyprotein gene of the PVY MSNR isolate is also available (Fellers et al., 2002, AF463399). Many partial sequences are also available in databases.
The genomic RNA of PVY is positive sense and approximately 9700 kb in length excluding the poly(A) tail (Shukla et al., 1994). As in all members of the picorna-like super-group, it is expressed as a large polyprotein precursor of 3063 amino acids for a PVYN isolate (Robaglia et al., 1989), 3061 amino acids for a PVYNTN isolate (Thole et al., 1993), and 3061 amino acids for a PVYO isolate (Singh & Singh, 1996). This is subsequently cleaved by proteases to yield nine functional proteins including those involved in RNA replication and other non-structural proteins (Shukla et al., 1994). In the 5'-3' direction, the nine proteins are referred as P1 (first protein), HC or HC-Pro (helper component protein), P3 (third protein), 6K1 (first 6 kDa protein), CI (cytoplasmic inclusion protein), 6K2 (second 6 kDa protein), NIa (small nuclear inclusion protein), NIb (large nuclear inclusion protein), and CP (coat protein) (Fig. 24).
There are two distal non-coding regions. The length of the 5' non-coding region is 184 nt (Robaglia et al., 1989; Singh & Singh, 1996). This includes blocks of sequences conserved in potyviruses, referred as the "potybox", box "a" (a 9 nt sequence, part of the potybox) and box "b". PVY has a potybox motif (UCAACACAACAU), in which 11/12 nucleotides match the consensus sequence, and a perfect copy of the consensus sequence motif of box b (UCAAGCAA). The potybox starts 12 nt from the 5'end and is separated from box b by a 39 nt sequence (Shukla et al., 1994). The 5' non-coding region is involved in initiation of translation (Levis & Astier-Manifacier, 1993). The length of the 3' non-coding region is variable: 326 nt (Van der Vlugt et al., 1989), 329-332 nt (Van der Vlugt et al., 1993), 333 nt (Rosner & Raccah, 1988); see a list compiled by Shukla et al. (1994).
The lengths (in number of amino acid residues) and positions of each of the gene products in the polyprotein on the basis of the sequence of the PVYN strain (Robaglia et al., 1989) are as follows :
| P1 : | 284 AA; | 1-284 |
| HC : | 456 AA; | 285-740 |
| P3 : | 365 AA; | 741 – 1105 |
| 6K1 : | 52 AA; | 1106- 1157 |
| CI : | 634 AA; | 1158 - 1791 |
| 6K2: | 52 AA; | 1792 - 1843 |
| NIa – VPg : | 188 AA; | 1844 - 2031 |
| NIa – Pro: | 244 AA; | 2032 - 2275 |
| NIb : | 521 AA; | 2276 – 2796 |
| CP : | 267 AA; | 2797 – 3063 |
NIa has a two-domain structure where the N-terminal domain is the genome-linked protein VPg. VPg is attached to the 5'end of the RNA via a phosphate ester linkage to Y60 in the conserved sequence motif NMY which is present in PVY NIa (Robaglia et al., 1989; Shukla et al., 1994). The full sequence of VPg was identified and a three-dimensionel model structure was proposed (Płochocka et al., 1996).
CP can be divided into three regions: a) a surface-exposed N-terminus of 30 amino acids, variable in length and sequence in different potyviruses; b) a core of 218 amino acids, highly conserved among potyviruses; and c) a surface-exposed C-terminus of 19 amino acids (Shukla & Ward, 1989). The N-terminal and C-terminal regions are not required for virus assembly. Trypsin digestion of particles removes these termini, but leaves a fully assembled particle (composed of the core region) that appears indistinguishable from untreated native particles by electron microscopy and is still infective (Shukla et al., 1988b).
Polyprotein processing as for all potyviruses involves three proteinases: NIa, HC and P3. The NIa proteinase cleavage sites were identified in the sequence of the PVYN strain (Robaglia et al., 1989) and further characterised recently (Rouis et al., 2001).
RNA synthesis is believed to occur in the cytoplasm. The entire RNA genome is copied. The replication complex comprises the proteins NIb, CI and VPg and possibly involves the proteins 6K1 and 6K2. The NIb protein is believed to be the RNA-dependent RNA polymerase since it contains the consensus sequence motif GDD found in viral RNA-dependant RNA polymerases, this motif being located at residues 2628-2630 (Robaglia et al., 1989).
As for all potyviruses, two proteins, HC and CP, are required for aphid transmission of PVY (see Transmission by Vectors). The N-terminal third of the HC protein contains a cluster of cysteine residues, similar to the consensus sequence of metal-binding sites in nucleic acid binding proteins (Robaglia et al., 1989). Indeed, the HC of PVY expressed in Escherichia coli possesses nucleic acid binding-activity which could be involved in cell-to-cell movement of the virus or in RNA replication (Robaglia et al., 1989; Maia & Bernardi, 1996). The N-terminal half of HC (amino acids 1 to 228) is capable of self-interaction, the 83 N-terminal residues being sufficient. Mutations in the conserved His and two Cys residues within the Cys-rich region of PVY HC-proteinase (amino acids 23 to 56) were reported to reduce self interaction (Urcuqui-Inchima et al., 1999a, 1999b). HC was shown to be involved in PVY accumulation in tobacco (Legavre et al., 1996), and to be a suppressor of post-transcriptional gene silencing (PTGS) (Brigneti et al., 1998). The HC protein, but not the 5' and 3' non-coding regions, P1 or CP, may be involved in induction of vein necrosis in tobacco (N. tabacum cv Xanthi) (Chachulska et al., 1997; Glais et al., 1998, 2002a).
VPg was shown to be involved in breaking resistance conferred by the gene va in tobacco (Masuta et al., 1999), by the gene pot-1 in tomato (Parella et al., 2002; Moury et al., 2004) and by the gene pvr2 in pepper (Moury et al., 2004). NIa was shown to interact with the resistance gene Ry in potato (Mestre et al., 2000), its protease activity however being not sufficient for elicitation of Ry (Mestre et al., 2003). NIb was found to be the elicitor of a veinal necrosis/hypersensitive response in root-knot nematode resistant tobacco cultivars (Fellers et al., 2002).
Relations with Cells and Tissues
Virions have been observed in the cytoplasm of infected cells, often closely associated with cytoplasmic inclusions, and within plasmodesmata (Weintraub et al., 1974). Rows of virus particles aligned on the Golgi apparatus, endoplasmic reticulum and around mitochondria of PVY infected cells have been described (Christie & Edwardson, 1977). Borges & David- Ferreira (1968) also reported mitochondria surrounded by filaments with a diameter of 9-10nm in Datura metel infected with a PVYN isolate. PVY was detected predominantly in vascular parenchyma cells rather than companion cells in minor veins in tobacco, pepper and tomato (Ding et al., 1998).
Most strains of PVY induce the formation of pinwheels, scrolls and short curved laminated aggregates in the cytoplasm of infected tissues (David-Ferreira & Borges, 1958; Skofenko et al., 1977; Mayee & Sarkar, 1982; Edwardson et al., 1984; Leseman, 1988; Edwardson & Christie, 1997). Such cylindrical inclusions (CIs) were shown to consist of a central tubule to which are attached 5-15 lamellae (Hiebert & McDonald, 1973) and are characteristic of Edwardson's subdivision IV potyviruses (Edwardson et al., 1984). They can often be observed adjacent to the cell nucleus. An Australian isolate has been reported to induce type I cylindrical inclusions (only pinwheels and scrolls) (Moghal & Francki, 1981; Edwardson et al., 1984). Purified CIs from infected plants consist of a single, non-glycosylated protein with a molecular weight of 67 kDa (Hiebert et al., 1971; Hiebert & McDonald, 1973; McDonald & Hiebert, 1974). The CI protein is serologically unrelated to virus coat protein or to host proteins or to the CIs of unrelated viruses (Hiebert et al., 1971; Purcifull et al., 1973). Serological analyses were reviewed by Hiebert et al. (1984b).
Cytoplasmic amorphous inclusions consisting of tubules and electron-opaque material are also induced by PVY. They stain reddish to magenta in Azure A (Christie & Edwardson, 1977). These inclusions are rod-like and morphologically dissimilar to those induced by other potyviruses (Christie & Edwardson, 1977; Leseman, 1988; Baunoch et al., 1990). HC protein was shown by immunogold labelling to be localized in these rod-like inclusions in tobacco (Baunoch et al., 1990).
Large crystalline cytoplasmic and nucleolar inclusions similar to those induced by other potyviruses have been reported in cells infected with two Brazilian isolates of PVY (Kitajima et al., 1968). PVY and PVA can be distinguished cytologically on the basis of the nucleolar inclusions (Edwardson & Christie, 1983).
The P1 protein of PVY was detected in association with CIs and in the cytoplasm of infected tobacco cells (Arbatova et al., 1998). HC is associated with amorphous inclusions which appear to be the primary site of HC accumulation in plant cells (Baunoch et al., 1990). NIa was shown to accumulate in both nucleus and cytoplasm (Rouis et al., 2001). PVY CP, HC and RNA were found within chloroplasts of infected tobacco leaves suggesting they may alter the chloroplastic function and play a role in symptom expression (Gunasinghe & Berger, 1991). Transgenic tobacco plants expressing PVY CP in their chloroplasts lose their green colour and display disrupted chloroplasts and cell structure (Naderi & Berger, 1997).
Changes in fresh matter content, photosynthesis and other metabolic activities associated with PVY multiplication were extensively studied in potato (Šindeláŕ et al., 1990) and tobacco leaves (Šindelářová et al., 2000; Ryšlavá et al., 2003).
Ecology and Control
Sources of infection. Initial infection and subsequent spread of PVY can originate from internal and/or external sources of infection. In potato crops, volunteers or groundkeepers, which may persist for several years, can be almost 100% infected with viruses such as PVY (De Bokx, 1987; Jones et al., 1996). Weeds such as Solanum dulcamara in Western Europe or Datura spp. in Mediterranean countries as well as nearby ware potato crops are often sources of infection. Potatoes, especially ground keepers or ware potatoes, are frequently reported as important sources for infection of other solanaceous crops (reviewed by Marte & Bellaza, 1988). Regarding tobacco crops, infected tomatoes were seen as an important source of inoculum for the outbreak in mid-1970s in North Carolina. Pepper transplants and seed potatoes were also identified as sources of inoculum as well as Physalis virginiana (perennial ground-cherry) (Gooding & Lapp, 1980). In tomato and pepper crops, solanaceous weeds, often primarily infected from potato crops, are the sources of infection in many countries: S. nigrum and S. dulcamara in Southern Europe (Marchoux et al., 1976; Gebre-Selassie et al., 1985, 1987); S. chacoense, S. nigrum and Physalis viscosa in South America (Pontis & Feldman, 1963; Ramallo et al., 1978; Vincente et al., 1979); S. gracile, S. nigrum, S. aculeatissimum, Physalis angulata, P. ciliosa and P. florida in Florida (Simons, 1956; Anderson, 1959); S. nodiflorum in Hawaii (Sakimura, 1953). In Canada (Ontario), PVYN could overwinter in Physalis heterophyla (perennial ground-cherry) and tomato could provide a seasonal amplification host for PVY which could then infect nearby potato or tobacco crops (Stobbs et al., 1994).
Control measures taken in seed potato production. Because of the importance of viruses, of which PVY is currently the most damaging in numerous countries, drastic control measures are taken for producing seed potatoes, including: planting of mother tubers with low levels of contamination, if not totally virus-free; planting in particular regions with low populations of vector aphids; isolation from ware crops and other sources of infection; eradication of weeds; field inspections and removal of any plant showing viral symptoms; treatments against aphids; early haulm destruction; post-harvest control; ELISA tests in the laboratory both during the vegetative period and in tubers or plantlets grown from the tubers (Oosterveld, 1987). Diagnosis based on visual inspections are not always reliable (Sturz et al., 1997). Serological tests based on a simple antigen-antibody reaction visualised after a few minutes (commercially available under the name "Pockett tests") are now also performed by inspectors in the field. These measures have proved to be highly effective in the main seed potato growing countries in the world. The PVYN strain has been the subject of specific eradication programmes in Canada (Singh, 1992; McDonald et al., 1994). Specific control measures have also been taken against PVYNTN in some countries, notably in France (Kerlan et al., 1997; Chatot et al., 1997).
Control of vector aphids in potato production. Because PVY can be acquired and transmitted within one minute, insecticide treatments are not sufficient to prevent its spread (Hille Ris Lambers et al., 1953), though they reduce it by limiting the populations of colonising aphids. Pyrethroids, such as deltamethrin, were shown to be effective (Gibson et al., 1982) and are more and more commonly used. In addition, mineral oils can be applied to reduce the spread of PVY significantly (Bradley et al., 1966; Wenzl & Foschum, 1973; Quéméner, 1976; Shands, 1977; Peters, 1987c; Merlet et al., 1996). Phytotoxic effects of mineral oils can be avoided (Schepers et al., 1984). Use of polymer webs to prevent PVY transmission by aphids in seed potatoes was experimentally tested with promising results (Harrewijn et al., 1991). The efficiency of such anti-vector treatments is enhanced by systems for monitoring aphids and forecasting virus spread. Sigvald (1992) produced a model for the spread of PVY in Sweden based on numbers of aphids caught in yellow traps, their relative efficiency as vectors, cultivar, and crop maturity in relation to susceptibility to PVY. In England, the model is based on catches of two species, Myzus persicae and Brachycaudus helichrysi (Harrington & Gibson, 1989). In the Netherlands, nine aphid species are taken into account because they appear in large numbers and are efficient vectors: Aphis fabae group, A. nasturtii, Brachycaudus spp., Macrosiphum euphorbiae, Myzus certus, M. persicae, Rhopalosiphum insertum and R. padi (De Bokx & Piron, 1990).
Breeding for resistance in potato. For reviews, see Ross (1986), Valkonen (1994), and Solomon-Blackburn & Barker (2001a, 2001b). Three types of resistance to PVY are used in potato breeding: resistance to infection, hypersensitivity and extreme resistance (Beekman, 1987). Resistance to infection or mature-plant resistance is a polygenic quantitative resistance related to the developmental stage of the plants and strongly depending on environmental conditions, notably the infection pressure and also on the viral strain (Beekman, 1987; Beemster, 1987; Gibson, 1991). Hypersensitivity and extreme resistance are based on single dominant genes designated as Ny and Ry, respectively. Rysto from S. stoloniferum and Ryadg from S. andigena have been identified by Cockerham (1970) and Munoz (1975), respectively. Both genes map on the North arm of potato chromosome XI (Hämäläinen et al., 1997; Brigneti et al., 1997; Hämäläinen et al., 1998; Gebhardt & Valkonen, 2001). Rysto conveys broad-spectrum extreme resistance to PVY, PVA and PVV (Barker, 1997). A third extreme resistance gene, from Solanum hougasii, currently designated Ryhou (Solomon-Blackburn & Barker, 2001a), was found by Cockerham (1970). Extreme resistance to PVY was also found in S. brevidens, S. etuberosum and S. fernandezianum (Valkonen et al., 1992) as well as in Solanum chacoense (Hosaka et al., 2001). Extreme resistance to PVYNTN was reported in twelve accessions of tuber-bearing wild Solanum species (Gaborjanyi et al., 2002). Extreme resistance is epistatic to hypersensitivity (Valkonen et al., 1994) and has been shown to be a form of hypersensitivity (Hinrichs et al., 1998). Hypersensitivity is strain specific. Genes Nc, Nytbr and Nz from S. tuberosum ssp. tuberosum protect against PVYC, PVYO and PVYZ, respectively (Cockerham, 1970; Jones, 1990). No commercial cultivar is known to be hypersensitive to PVYN, except maybe the Polish cultivar Rywal (Chrzanowska & Kerlan, unpublished), though hypersensitivity against PVYN was found in various Solanum species (Valkonen, 1997). The gene Nytbr maps to chromosome IV (Celebi-Toprak et al., 2002). Nydms from S. demissum and Nychc from S. chacoense have also been reported (Cockerham, 1970). The genetic basis of the leaf-drop symptom involves, in addition to the gene Ny, another locus that modifies at high temperatures the hypersensitivity triggered by Ny (Valkonen et al., 1998). The gene Y-1 involved in the hypersensitivity reaction has been cloned and is located on the potato chromosome XI (Vidal et al., 2002).
Attempts to introgress extreme resistance to PVY have been made since 1944 and, although most breeding lines bearing the gene Rysto are male-sterile, numerous Ry cultivars (at least 27) have been produced in the Netherlands, Germany, Poland and Hungary. This resistance has proved to be quite durable (Solomon-Blackburn & Barker, 2001b). Resistance to infection by PVY is also largely used in potato breeding, though it is difficult to select and requires expansive and laborious field trials (Pérennec, 1982). Marker-assisted selection for resistance to PVY has been applied to the gene Ryadg (Hämäläinen et al., 1997). SCAR markers (Kasai et al., 2000) and a bacterial artificial chromosome library (Zhang et al., 2003) were developed for this gene. RAPD markers were reported for a non-identified gene from S. chacoense conferring extreme resistance (Hosaka et al., 2001) and for the gene Nytbr (Celebi-Toprak et al., 2002). Genetically engineered resistance using either the CP gene, the P1 gene or the NIb gene of PVY was used to generate resistant transgenic plants from commercial potato cultivars (Lawson et al., 1990; Kaniewski et al., 1990; Farinelli et al., 1992; Malnoë et al., 1994; Pehu et al., 1995; Okamoto et al., 1996; Mäki-Valkama et al., 2000; Racman et al., 2001; Schubert et al., 2004). CP-mediated resistance was described as extreme resistance with often a broad spectrum. Protection against PVY infections in potato was also obtained by using genomic constructs deriving from other potyviruses (Tacke et al., 1996; Kerlan et al., 1999b). Transformed versions of established cultivars Russett Burbank and Shepody were registered in USA as being resistant to PVY (Solomon-Blackburn & Barker, 2001b).
Control measures in tobacco production. In tobacco production, breeding for resistance is the main control method against PVY. The genotype Virginia A mutant (Koelle, 1958) as well as many European and American commercial cultivars, such as cvs Paraguay, Virginie SCR and Havana 307, display noticeable levels of resistance (De Baets & Slaats, 1963; Gooding et al., 1985; Ano et al., 1995), most of the genes being recessive (Koelle, 1961; Gupton & Burk, 1973), often allelic forms at a single locus (Wernsman, 1991; Yamamoto, 1992). The gene va has been extensively utilized in breeding programs (Yamasaki et al., 1992). However, introgression of resistance is often associated with decrease of yield and cured leaf quality (Kozumplik et al., 1996). Resistance conferred by va is due to deletions at the Va locus (Noguchi et al., 1999). In many countries, especially where early infection is a problem, seed beds must be protected by fleece and additional measures taken such as isolation from potato, tomato and pepper crops, and eradication of weeds (Blancard, 1998). Latore & Flores (1985) also suggested that mild strains of PVY could be used to protect an annual crop such as tobacco against PVY in Chile.
Pathogen-derived resistance, mainly from expression of the PVY coat protein, has been extensively studied in tobacco, although more for investigating the mechanisms of protection (Van der Vlugt et al., 1992; Van der Vlugt & Goldbach, 1993; Kollar et al., 1993; Farinelli & Malnoë, 1993; Vardi et al., 1993; Dinant et al., 1993; Audy et al., 1994; McDonald et al., 1997a; Han et al., 1999; Guo et al., 2003) than in order to use this strategy in commercial tobacco cultivars (Young et al., 1995; Sudarsono et al., 1995).
Control measures in tomato and pepper production. Physical barriers such as sticky yellow polyethylene sheets and coarse white nets were used for controlling spread of PVY in pepper crops in Israel (Cohen & Marco, 1973; Cohen, 1981). Use of oil sprays is commonplace in pepper crops in Florida (Falk & Duffus, 1988). Wild tomato Lycopersicon hirsutum PI 247087 was found to be resistant (immune) to four Australian PVY isolates (Thomas, 1981; Thomas & MacGrath, 1988) and this resistance proved to be effective against a large range of PVY isolates (Legnani et al., 1995). Breeding for resistance in pepper has been undertaken for a long time (Cook & Anderson, 1959). Many recessive or dominant resistance genes and QTLs have been identified: pot-1 from L. hirsutum located on chromosome T3 of tomato; pvr1, pvr2 and pvr5 from Capsicum annuum located on chromosome P4 of pepper; Pvr4 from C. annuum and Pvr7 from C. chinense, both located on chromosome P10 of pepper (Kyle & Palloix, 1997; Caranta et al., 1997; Grube et al., 2000; Parella et al., 2002). The pvr2 gene corresponds to the eukaryotic initiation factor 4E (Ruffel et al., 2002). PCR markers are available for some genes (e.g. Pvr7) and are used by breeders. Recessive resistance genes, especially alleles pvr21 and pvr22, have been introgressed into numerous European and American pepper cultivars. The dominant gene Pvr4 conferring resistance to all PVY strains has been recently introgressed into commercial hybrids (Palloix, pers. comm.).
Notes
Molecular detection. Numerous molecular assays were developed for PVY detection in leaves, potato tubers and aphids (reviewed by Singh, 1999) including cDNA hybridization (De Bokx & Cuperus, 1987; Dhar & Singh, 1994; Singh & Singh, 1995; Baulcombe & Fernandez-Northcote, 1988), RT-PCR (Barker et al., 1993; Singh et al., 1996; Singh, 1998; Singh et al., 1999; Boonham et al., 2002a), RT-PCR coupled to a colorimetric reaction (Hataya et al., 1994; Chandelier et al., 2001; Nicolaisen et al., 2001) or a fluorescent label (Walsh et al., 2001), print-capture PCR (Varveri, 2000), and multiplex RT-PCR for detection of PVY in mixed infections (Boonham et al., 2000; Nie & Singh, 2000; Klerks et al., 2001). All these asays were found 1000 to 10000 times more sensitive than ELISA. Microarray technology was recently applied to PVY detection (Boonham et al., 2003).
Taxonomy. Genomic variability of PVY has been extensively studied (reviewed by Glais et al., 2002b) leading to the classification of PVY isolates into two, three or four clusters (O, N, NP and C2), in rather good agreement with classical PVY strain definitions based on host range and symptomatology. However most classifications obtained from phylogenetic analyses probably rely on neutral markers and correlate only partially with biological classifications. For instance, PVYC isolates were split into two genetic lineages, though the classical PVYC strain is a single pathotype (Blanco-Urgoiti et al., 1998b). In contrast, pepper-infecting PVY isolates were shown to be a single genetic lineage containing several pathotypes (Romero et al., 2001). There are many exceptions, such as reference isolates PVYN-Fr and PVY MSMR (a tobacco isolate), which were grouped with the potato PVYO isolates (Blanco-Urgoiti et al., 1996). Recombination events may also result in flawed if not false molecular classifications based on the capsid protein alone, as was shown for the PVYN-W and PVYN:O isolates (Glais et al., 1998; Singh et al., 2003) and a pepper isolate (Fakhfakh et al., 1995). Lastly, the exact positions and outlines of different groups of isolates have yet to be precisely defined, as for instance the exact relationship between the potato (PVYN) and tobacco (NSNR, MSNR) necrotic strains (Sudarsono et al., 1993: McDonald & Kristjansson, 1993), or the border between tuber-necrosing (PVYNTN) and non-necrosing PVYN isolates. A recombination breakpoint in the coat protein gene was thought to correlate with the potato tuber necrotic ringspot phenotype induced by the PVYNTN isolates (Revers et al., 1996; Glais et al., 1998; Boonham et al., 1999), but it is not present in all isolates associated with PTNRD (Ohshima et al., 2000; Glais et al., 2001; Boonham et al., 2002b).
Differentiation and consequently detection of the various groups of isolates are often uncertain. For instance, numerous RT-PCR methods have been published for detection of PVYNTN isolates (Weidemann & Maiss, 1996; Glais et al., 1996; Weilgunny & Singh, 1998; Singh et al., 1998; Rosner & Maslenin, 1999a, 1999b; Boonham et al., 2002a; Szemes et al., 2002; Nie & Singh, 2002b, 2003a; Moravec et al., 2003), but they are often outdated or even unreliable, being based on small numbers of isolates or inadequately characterised isolates and also relying on gene sequences not proved to be responsible for inducing tuber necrosis. Bioassays, in which PVY isolates are inoculated to sensitive potato cultivars, could be another means to discriminate PVYNTN from the rest of PVYN. However, the reliability of such bioassays when carried out under artificial conditions is also in doubt as shown by some experiments (Kerlan & Tribodet, 1996; McDonald & Singh, 1996b).
Evolution. Several steps in PVY history can be recognized, e.g. the emergence of the PVYN strain in potato and tobacco, of PVYN variants in potato, and of pathotypes breaking recessive resistance genes in tobacco and in pepper. Both PVYO and PVYN strains are thought to originate from the Andean countries where they were probably adapted to infection of various wild Solanum species in their natural habitats, maybe in separate regions (Silberschmidt, 1960; Brücher, 1969; Jones, 1981; Valkonen, 1997). PVYO and PVYN could have diverged from a common viral ancestor that has followed two different evolutionary paths (Glais et al., 2002b), maybe on different hosts (either Solanum sp. or Nicotiana sp.). Indeed, from data regarding the biological and genetic specificity of pepper PVY isolates, it was inferred that the host seems to be an important factor in PVY evolution and that the strain specialization in the virus could be an effect of co-evolution with the plant (Romero et al., 2001).
Advances in knowledge of genomic diversity of PVY have provided more and more insights into PVY evolution. Links between mutation or recombination events in the genome and evolution of PVY have been thoroughly studied (Matousek et al., 2000; Moury et al., 2002; Glais et al., 2002b; Nie & Singh, 2003b). From phylogenetic analysis based on the P1 protein gene and the full genomic RNA sequence, it was suggested that North American-PVYN (NA-PVYN) and European-PVYN (Eu-PVYN) evolved in parallel, and that NA-PVYNTN originated from NA-PVYN by mutations, whereas Eu-PVYNTN evolved from Eu-PVYN and PVYO by recombination (Nie & Singh, 2003b).
Figures

Mottle and rugosity in leaflets of a naturally infected plant of potato cv Monalisa. Photograph: Camille Kerlan, INRA, France.

Severe mottle and and leaf distortion in leaflets of a naturally infected plant of potato cv Mana. Photograph: Camille Kerlan, INRA, France.

Typical yellowing and yellow-green ringspots in a lower leaf of potato cv Nicola. Photograph: Karine Charlet-Ramage, INRA-GNIS, France.

Necrotic patterns in a lower leaf of potato cv Nicola, symptoms frequently linked to infection by PVYNTN isolates. Photograph: Karine Charlet-Ramage, INRA-GNIS, France.

Severe necrosis in lower leaflets of a naturally infected potato plant. Photograph: Karine Charlet-Ramage, INRA-GNIS, France.

Leaf drop symptom and collapse of a potato plant. Photograph: Karine Charlet-Ramage, INRA-GNIS, France.

Secondary infection by a PVYO isolate in a potato field: severe mottle and crinkling. Photograph: Yves Le Hingrat, FNPPPT, France Courtesy of FNPPPT & GIS.
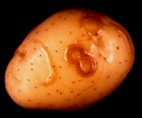
Potato tuber necrotic ringspot disease: brown necrotic ringspots and faint colourless curved swelling of the tuber skin (cv Nicola). Photograph: Karine Charlet-Ramage, INRA-GNIS, France.

Potato tuber necrotic ringspot disease: rings on tubers of cv Monalisa. Photograph: K. Charlet-Ramage, INRA-GNIS, France.

Dark-brown arcs and rings with severe depression of the periderm (last stage of potato tuber necrotic ringspot disease expression) on a progeny tuber of a breeding line. Photograph: K. Charlet-Ramage, INRA-GNIS, France.

Potato tuber necrotic ringspot disease: external necrotic rings on tubers of cv Rua. Photograph: John Fletcher, ICFR, New Zealand.

Potato tuber necrotic ringspot disease: internal spots in tubers of cv Rua. Photograph: John Fletcher, ICFR, New Zealand.

Field infection of Virginia tobacco by PVY: multitude of coalescent yellow spots becoming necrotic. Photograph: Dominique Blancard, INRA, France.

Chlorotic patterns associated with PVY infection in a tomato crop. Photograph: Dominique Blancard, INRA, France.

Yellow spots and vein necrosis induced by PVY in pepper. Photograph: Dominique Blancard, INRA, France.

Interveinal clearing in Nicotiana tabacum cv Xanthi inoculated with a PVYO isolate. Photograph: Laurent Glais, INRA, France.

Typical vein necrosis, puckering and leaf bending in Nicotiana tabacum cv Xanthi inoculated with a PVYN isolate. Photograph: Laurent Glais, INRA, France.

Necrotic local lesions in potato cv Desiree inoculated with a PVYO isolate. Photograph: Camille Kerlan, INRA, France.

Systemic hypersensitivity in potato cv Eersteling inoculated with a PVYC isolate. From Kerlan et al., 1999a.

Necrotic local lesions in potato cv Maris Bard inoculated with a PVYC isolate. From Kerlan et al., 1999a.

Tuber necrosis on potato cvs Nicola (left), Nadine (top middle), Hermes (right) inoculated with a PVYNTN isolate. Photograph: Isla Browning, SASA, UK Crown Copyright, SASA, Edinburgh, UK.

Local lesions in Chenopodium amaranticolor inoculated with either a PVYO or a PVYC isolate. Photograph: Jean-Pierre Cohan, INRA, France.

Schematic map of the genomes of four groups of PVY potato isolates: PVYN, PVYNTN, PVYNW, PVYO. From Glais et al., 2002a.

The pea aphid, Acyrthosiphon pisum (apterous adult), one of of the species that transmits PVY. Photograph: Joelle Rouzé-Jouan, INRA, France.

Myzus persicae (winged adult), the main vector aphid species of PVY. Photograph: Bernard Chaubet, INRA, France.
References list for DPV: Potato virus Y (414)
- Abdallah, Desjardins & Dodds, Plant Disease 75: 1019, 1991.
- Anderson, Phytopathology 49: 97, 1959.
- Ano, Blancard & Cailleteau, Annales du Tabac, Seita 2-27: 35, 1995.
- Arbatova, Lehto, Pehu & Pehu, Journal of General Virology 79:2319, 1998.
- Aubert, Proceedings of the 2nd International Scientific Tobacco Congress, Brussels: 83, 1958.
- Audy, Palukaitis, Slack & Zaitlin, Molecular Plant-Microbe Interactions 7: 15, 1994.
- Bala & Bargava, Zeitschrift fur Pflanzenkrankheiten und Pflanzenschutz 84: 342, 1977.
- Banttari & Goodwin, Plant Disease 69: 202, 1985.
- Banttari, Ellis & Khurana, in Potato Health Management, p.127, ed. R.C. Rowe, St. Paul, USA: APS Press, 1993.
- Barker, Theoretical and Applied Genetics 95: 1258, 1997.
- Barker, Webster & Reavy, Potato Research 36: 13, 1993.
- Bartels, Phytopathologische Zeitschrift 49: 257, 1964.
- Baulcombe & Fernandez-Northcote, Plant Disease 72: 307, 1988.
- Baunoch, Das & Hari, Journal of General Virology 71: 2479, 1990.
- Bawden & Kassanis, Annals of Applied Biology 34: 503, 1947.
- Bawden & Kassanis, Annals of Applied Biology 38: 402, 1951.
- Beczner, Horvath, Romhanyi & Forster, Potato Research 27: 339, 1984.
- Beekman, in Viruses of potatoes and seed-potato production, 2nd edition, p.162, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987.
- Beemster, in Viruses of potatoes and seed-potato production, 2nd edition, p. 116, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987.
- Beemster & De Bokx, in Viruses of potatoes and seed-potato production, 2nd edition, p. 84, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987.
- Bellardi, Rubiesautonell & Vicchi, Acta Horticulturae 432: 306, 1996.
- Bercks, Virology 12: 311, 1960.
- Berger, Thornbury & Pirone, Journal of Virological Methods 12: 31, 1985.
- Bhat, Varma, Pappu, Rajamannar, Jain & Praveen, Plant Pathology 48: 648, 1999.
- Blanc, Ammar, Garcia-Lampasona, Dolja, Llave, Baker& Pirone, Journal of General Virology 79: 3119, 1998.
- Blancard, in Maladies du Tabac, p. 325, ed. D. Blancard, Paris: INRA, 1998.
- Blancard, Ano & Cailleteau, Annales du Tabac, Seita 2-27: 43, 1995.
- Blanco-Urgoiti, Sanchez, Dopazo & Ponz, Archives of Virology 141: 2425, 1996.
- Blanco-Urgoiti, Tribodet, Leclere, Ponz, Pérez de San Román, Legorburu & Kerlan, European Journal of Plant Pathology 104 : 811; 1998a.
- Blanco-Urgoiti, Sánchez, Pérez de San Román, Dopazo & Pons, Journal of General Virology 79: 2037, 1998b.
- Boonham & Barker, Journal of General Virology 74: 193, 1998.
- Boonham, Hims, Barker & Spence, European Journal of Plant Pathology 105: 617, 1999.
- Boonham, Walsh, Mumford & Barker, European Plant Protection Organization Bulletin 30: 427, 2000.
- Boonham, Walsh, Preston, North, Smith & Barker, Journal of Virological Methods 102: 103, 2002a.
- Boonham, Walsh, Hims, Preston, North & Barker, Plant Pathology 51: 117, 2002b.
- Boonham, Walsh, Smith, Madagan, Graham & Barker, Journal of Virological Methods 108: 181, 2003.
- Borges & David-Ferreira, Revista Biologia 6: 421, 1968.
- Bradley, Moore & Pond, Nature 209: 1370, 1966.
- Brigneti, Garcia-Mas & Baulcombe, Theoretical and Applied Genetics 94: 198, 1997.
- Brigneti, Voinnet, Li, Ji, Ding & Baulcombe, The EMBO Journal 17: 6739, 1998.
- Browning, Charlet, Chrzanowska, Dedic, Kerlan, Kryszczuk, Schubert, Varveri, Werkman & Wolf, Abstracts of the 12th EAPR Virology Section Meeting, Rennes: 51, 2004.
- Brücher, Angewandte Botanik 43: 241, 1969.
- Brunt, Kenten & Philips, Annals of Applied Biology 88: 115, 1978.
- Calvert, Cooper & McClure, Record of Agricultural Research, Northern Ireland Department of Agriculture 28: 63, 1980.
- Canto, Ellis, Bowler & Lopez-Abella, Plant Disease, 79: 234, 1995a.
- Canto, Lopez-Moya, Serra-Yoldi, Diaz-Ruiz & Lopez-Abella, Phytopathology 85: 1519, 1995b.
- Caranta, Lefebvre & Palloix, Molecular Plant-Microbe Interactions 10: 872, 1997.
- Carlebach, Raccah & Loebenstein, Annals of Applied Biology 101: 511, 1982.
- Celebi-Toprak, Slack & Jahn, Theoretical and Applied Genetics 104: 669, 2002.
- Čeŕovská, Plant Pathology 47: 505, 1998.
- Chachulska, Chrzanowska, Robaglia & Zagorski, Archives of Virology 142: 765, 1997.
- Chandelier, Dubois, Baden, De Leener, Warmon, Remacle & Lepoivre, Journal of Virological Methods 91: 99, 2001.
- Chatot, Kerlan, Ramage & Urvoy, Proceedings of 10th Congress of the Mediteranean Phytopathological Union: 669, 1997.
- Chirkov, Olovnikov, Surguchyova & Atabekov, Annals of Applied Biology 104: 477, 1984.
- Chiu, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 387, ed. R. G. Milne, New York: Plenum Press, 1988.
- Christie & Edwardson, Florida Agricultural Experiment Station Monograph Series 9: 1977.
- Chrzanowska, Phytopathologica Polonica 8: 15, 1994.
- Clark & Hill, Phytopathology News 12: abstract 203, 1978.
- Cockerham, Annals of Applied Biology 30: 338, 1943.
- Cockerham, Heredity 25: 309, 1970.
- Cohen, Phytoparasitica 9: 69, 1981.
- Cohen & Marco, Phytopathology 63: 1207, 1973.
- Cohen, Loebenstein & Milne, Journal of Virological Methods 4: 323, 1982.
- Cook & Anderson, Phytopathology 49: 198, 1959.
- Croslin, Hamm, Eastwell, Thornton, Brown, Corsini, Shiel & Berger, Plant Disease 86: 1177, 2002.
- Damirdagh & Shepherd, Phytopathology 60: 132, 1970.
- d'Aquino, Dalmay, Burgyan, Ragozzino & Scala, Plant Disease 79: 1046, 1995.
- David-Ferreira & Borges, Boletim Sociedade Broteriana 32: 329, 1958.
- De Baets & Slaats, Proceedings 3rd International Scientific Congress, Salisbury: 300, 1963.
- De Bokx, in Viruses of potatoes and seed-potato production, 2nd edition, p.58, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987.
- De Bokx & Cuperus, European Plant Protection Organization Bulletin 17: 73, 1987.
- De Bokx & Huttinga, CMI/AAB Descriptions of Plant Viruses 242: 1981.
- De Bokx & Piron, Abstracts of the 7th Triennal Conference of the European Association for Potato Research, Warsaw: 244, 1978.
- De Bokx & Piron, Netherlands Journal of Plant Pathology 96: 237, 1990.
- De Bokx, Kractchanova & Maat, Potato Research 18: 38, 1975.
- De Bokx, Van Hoof & Piron, Netherlands Journal of Plant Pathology 84: 95, 1978.
- De Bokx, Piron & Cuperus, Potato Research 24: 36, 1981.
- Delgado-Sanchez & Grogan, Phytopathology 56: 1397, 1966.
- Delon, Schiltz & Bardon, Annales du Tabac, Seita 2-29: 1, 1997.
- De Wijs & Suda-Bachman, Netherlands Journal of Plant Pathology 85: 23, 1979.
- Dhar & Singh, Journal of Virological Methods 50: 197, 1994.
- Dinant, Blaise, Kusiak, Astier-Manifacier & Albouy, Phytopathology 83: 918, 1993.
- Ding, Carter, Deom & Nelson, Plant Physiology 116: 125, 1998.
- Dougherty & Carrington, Annual Reviews of Phytopathology 26: 123, 1988.
- Draper, Pasche & Gudmestad, American Journal of Potato Research 79: 155, 2002.
- Edwardson & Christie, Phytopathology 73: 290, 1983.
- Edwardson & Christie, Florida Agricultural Experiment Station Monograph Series 18-II: 467, 1997.
- Edwardson, Christie & Ko, Phytopathology 74: 1111, 1984.
- Ellis, Stace-Smith, Bowler & Mackenzie, Canadian Journal of Plant Pathology 18: 64, 1996.
- Ellis, Stace-Smith & de Villiers, Plant Disease 81: 481, 1997.
- Fakhfakh, Makni, Robaglia, Elgaaied & Marrakchi, Agronomie 15: 569, 1995.
- Falk & Duffus, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 275, ed. R. G. Milne, New York: Plenum Press, 1988.
- Farinelli & Malnoë, Molecular Plant-Microbe Interactions 6: 284, 1993.
- Farinelli, Malnoë & Collet, Bio/Technology 10: 1020, 1992.
- Fauquet & von Wechmar, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 379, ed. R. G. Milne, New York: Plenum Press, 1988.
- Fellers, Tremblay, Handest & Lommel, Molecular Plant Pathology 3: 145, 2002.
- Fernandez-Northcote & Gugerli, Fitopatologia 22: 33, 1987.
- Fletcher, New Zealand Journal of Crop and Horticultural Science 17: 259, 1989.
- Fletcher, New Zealand Journal of Crop and Horticultural Science 29: 213, 2001.
- Fribourg & Nakashima, Phytopathology 74: 1363, 1984.
- Fritzsche, Schmelzer & Schmidt, Archiv für Pflanzenschutz 3: 89, 1967.
- Gaborjanyi, Horvath, Kazinczi & Takacs, Proceedings of the APLS-ADRIA Scientific Workshop, Opatija: 59, 2002.
- Gebhardt & Valkonen, Annual Reviews of Phytopathology 39: 79, 2001.
- Gebre-Selassie, Marchoux, Delecolle & Pochard, Agronomie 5: 621, 1985.
- Gebre-Selassie, Marchoux, Laterrot & Blancard, PHM –Revue Horticole 281: 43, 1987.
- Gibson, Potato Research 34: 205, 1991.
- Gibson, Rice & Sawicki, Annals of Applied Biology 100: 49, 1982.
- Glais, Kerlan, Tribodet, Marie-Jeanne Tordo, Robaglia & Astier-Manifacier, European Journal of Plant Pathology 102: 655, 1996.
- Glais, Tribodet, Gauthier, Astier-Manifacier, Robaglia & Kerlan, Archives of Virology 143: 2077, 1998.
- Glais, Tribodet & Kerlan, Proceedings of the 11th EAPR Virology Section Meeting, Havlickuv Brod-Trest: 70, 2001.
- Glais, Tribodet & Kerlan, Archives of Virology 147: 363, 2002a.
- Glais, Kerlan & Robaglia, in Plant viruses as molecular pathogens, p. 225, ed. J.A. Khan & J. Dijkstra, Binghampton NY, USA: The Haworth Press, 2002b.
- Gnutova & Krylov, Acta Phytopathologica Academiae Scientiarum Hungaricae 10: 185, 1975a.
- Gnutova & Krylov, Acta Phytopathologica Academiae Scientiarum Hungaricae 10: 203, 1975b.
- Gooding, Tobacco Science 29: 99, 1985.
- Gooding & Lapp, Tobacco Science 24: 91, 1980.
- Gooding & Tolin, Plant Disease Reporter 57: 200, 1973.
- Gooding & Tsakiridis, Phytopathology 61: 943, 1971.
- Gooding, Wernsmann & Rufty, Tobacco Science 29: 32, 1985.
- Goodman, McDonald, Horne & Bancroft, Philosophical Transactions of the Royal Society of London, Series B 276: 173, 1976.
- Govier & Kassanis, Virology 57: 285, 1974a.
- Govier & Kassanis, Virology 61: 420, 1974b.
- Govier, Kassanis & Pirone, Virology 78: 306, 1977.
- Grube, Blauth, Arnedo-Andres, Caranta & Jahn, Theoretical and Applied Genetics 101: 852, 2000.
- Gugerli, Phytopathologische Zeitschrift 92: 51, 1978.
- Gugerli, Journal of Virological Methods 9: 249, 1984.
- Gugerli & Fries, Journal of General Virology 64: 2471, 1983.
- Gugerli & Gehriger, Potato Research 23: 353, 1980.
- Gunasinghe & Berger, Molecular Plant-Microbe Interactions 4: 452, 1991.
- Guo, Han, Zhang & Wang, Shi Yan Sheng Wu Xue Bao 36: 176, 2003.
- Gupton & Burk, Journal of Heredity 64: 289, 1973.
- Hämäläinen, Watanabe, Valkonen, Arihara, Plaisted, Pehu, Miller & Slack, Theoretical and Applied Genetics 94: 192, 1997.
- Hämäläinen, Sorri, Watanabe, Gebhardt & Valkonen, Theoretical and Applied Genetics 96: 1036, 1998.
- Hammond & Lawson, Journal of Virological Methods 20: 203, 1988.
- Han, Cho, You, Nam, Park, Shin, Park & Paek, Molecules and Cells 9: 376, 1999.
- Harrewijn, den Ouden & Piron, Entomologia Experimentalis et Applicata 58: 101, 1991.
- Harrington & Gibson, Potato Research 32: 167, 1989.
- Harrington, Katis & Gibson, Potato Research 29: 67, 1986.
- Harrison, Finch, Gibbs, Hollings, Shepherd, Valenta & Wetter, Virology 45: 356, 1971.
- Hataya, Inoue & Shikata, Journal of Virological Methods 46: 223, 1994.
- Heath, Sward, Moran, Mason & Hallam, Australian Journal of Agricultural Research 38: 395, 1987.
- Hiebert & McDonald, Virology 56: 349, 1973.
- Hiebert & Purcifull, in Potyvirus Taxonomy, p.321, ed. O.W. Barnett, Wien & New-York: Springer, 1992.
- Hiebert, Purcifull, Christie & Christie, Virology 43: 638, 1971.
- Hiebert, Thornbury & Pirone, Virology 135: 1, 1984a.
- Hiebert, Purcifull & Christie, in Methods in Virology, vol. VIII, p. 225, ed. K. Maramorosch & H. Koprowski, Orlando: Academic Press, 1984b.
- Hille Ris Lambers, Reestman & Schepers, Netherlands Journal of Agricultural Science 1: 188, 1953.
- Hinostroza-Orihuela, Virology 67: 276, 1975.
- Hinrichs, Berger & Shaw, Journal of Virological Methods 66: 195, 1997.
- Hinrichs, Berger & Shaw, Journal of General Virology 79: 167, 1998.
- Horvath, Acta Phytopathologica Academiae Scientiarum Hungaricae 1: 125, 1966a.
- Horvath, Acta Phytopathologica Academiae Scientiarum Hungaricae 1: 333, 1966b.
- Horvath, Acta Phytopathologica Academiae Scientiarum Hungaricae 2: 95, 1967a.
- Horvath, Acta Phytopathologica Academiae Scientiarum Hungaricae 2: 195, 1967b.
- Hosaka, Hosaka, Mori, Maida & Matsunaga, American Journal of Potato Research 78: 192, 2001.
- Huttinga, Netherlands Journal of Plant Pathology 79: 125, 1973.
- Huttinga, Netherlands Journal of Plant Pathology 81: 58, 1975.
- Huttinga & Maat, in Viruses of potatoes and seed-potato production, 2nd edition, p.25, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987.
- Huttinga & Mosch, Netherlands Journal of Plant Pathology 80: 19, 1974.
- Inouye, Iizuka & Kameya-Iwaki, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 393, ed. R. G. Milne, New York: Plenum Press, 1988.
- Jakab, Droz, Brigneti, Baulcombe & Malnoë, Journal of General Virology 78: 3141, 1997.
- Jeffries, in FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm 19, p. 70, Rome: Food and Agriculture Organization of the United Nations / International Plant Genetic Resources Institute, 1998.
- Jones, in Pests, pathogens and vegetation, p.89, ed. J.M. Thresh, London: Pitman Books, 1981.
- Jones, Annals of Applied Biology 117: 93, 1990.
- Jones & Fribourg, CMI/AAB Descriptions of Plant Viruses 316: 1986.
- Jones, Woodford, Main, Pallett & Barker, Annals of Applied Biology 129: 471, 1996.
- Jordan & Hammond, Journal of General Virology 72: 25, 1991.
- Kaniewski, Lawson, Sammons, Haley, Hart, Delannay & Tumer, Bio/Technology 8: 750, 1990.
- Kasai, Morikawa, Sorri, Valkonen, Gebhardt & Watanabe, Genome 43: 1, 2000.
- Kassanis & Govier, Journal of General Virology 13: 221, 1971.
- Katis & Gibson, Potato Research 28: 65, 1985.
- Katis, Carpenter & Gibson, Plant Pathology 35: 152, 1986.
- Kennedy, Day & Eastop, A conspectus of aphids as vectors of plant viruses, London: Commonwealth Institute of Entomology, 1962.
- Kerlan & Tribodet, Abstracts of the 13th Triennal Conference of the European Association for Potato Research, Veldhoven: 65, 1996.
- Kerlan, Robert, Pérennec & Guillery, Potato Research 30: 651, 1987.
- Kerlan, Ramage, Hullé & Tribodet, Proceedings of the 10th Congress of the Mediteranean Phytopathological Union, Montpellier: 669, 1997 .
- Kerlan, Tribodet, Glais & Guillet, Journal of Phytopathology 147: 643, 1999a.
- Kerlan, Chauvin, Tribodet & Le Dantec, Abstracts of the 14th Triennal Conference of the European Association for Potato Research, Sorrento: 700, 1999b.
- Kitajima, de Camargo & Costa, Journal of Electron Microscopy 17: 141, 1968.
- Klerks, Leone, Verbeek, van den Heuvel & Schoen, Journal of Virological Methods 93: 115, 2001.
- Klinkowski & Schmelzer, Phytopathologische Zeitschrift 28: 285, 1957.
- Koelle, Proceedings 2nd International Scientific Tobacco Congress, Brussels: 122, 1958.
- Koelle, Der Zuchter 31: 71, 1961.
- Kollar, Thole, Dalmay, Salamon & Balázs, Biochimie 75: 623, 1993.
- Kostiw, Potato Research 18: 637, 1975.
- Kozumplik, Boic, Nemcevic & Pejic, Annales du Tabac, Seita 2-28: 29, 1996.
- Kyle & Palloix, Euphytica 97: 183, 1997.
- Latore & Flores, Plant Disease 68: 884, 1984.
- Latore & Flores, Plant Disease 69: 930, 1985.
- Latore, Andrade, Penaloza, & Escaffi, Plant Disease 66: 893, 1982.
- Lawson, Kaniewski, Haley, Rozman, Newell, Sanders & Turner, Bio/Technology 8: 127, 1990.
- Lecoq, Lot, Kleinhampel & Kegler, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 349, ed. R. G. Milne, New York: Plenum Press, 1988.
- Legavre, Maia, Casse-Delbart, Bernardi & Robaglia, Journal of General Virology 77: 1343, 1996.
- Legnani, Euphytica 86: 219, 1995.
- Legnani, Gebre Selassie, Nono Womdim, Gognalons, Moretti, Latterot & Marchoux, Euphytica 86: 219, 1995.
- Leiser & Richter, Archiv für Phytopathologie und Pflanzenschutz 14: 337, 1978.
- Le Romancer, Kerlan & Nédellec, Plant Pathology 43: 138, 1994.
- Lesemann, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 179, ed. R. G. Milne, New York: Plenum Press, 1988.
- Levis & Astier-Manifacier, Virus Genes 7: 367, 1993.
- Li, Li & Wang, Plant Disease 85: 447, 2001.
- Liu, Sukhacheva, Erokhina & Schubert, European Journal of Plant Pathology 105: 389, 1999.
- Llave, Martínez, Díaz-Ruíz & López-Abella, European Journal of Plant Pathology 105: 847, 1999a.
- Llave, Martínez, Díaz-Ruíz & López-Abella, Phytopathology 89: 1176, 1999b.
- Lopez-Abella, Bradley & Harris, Advances in Disease Vector Research 5: 251, 1988.
- Lopez-Moya, Canto, Diaz-Ruiz & Lopez-Abella, Journal of General Virology 76: 2293, 1995.
- Maat & Huttinga, in Viruses of potatoes and seed-potato production, 2nd edition, p.45, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987.
- Maia & Bernardi, Journal of General Virology 77: 869, 1996.
- Maia, Haenni & Bernardi, Journal of General Virology 77: 1335, 1996.
- Mäki-Valkama, Valkonen, Kreuze & Pehu, Molecular Plant-Microbe Interactions 13: 366, 2000.
- Makkouk & Gumpf, Phytopathology 64: 1115, 1974.
- Makkouk & Gumpf, Virology 63: 336, 1975.
- Makkouk & Gumpf, Phytopathology 66: 576, 1976.
- Malnoë, Farinelli, Collet & Reust, Plant Molecular Biology 25: 963, 1994.
- Marchoux, Gebre & Quiot, Agriculturae Conspectus Scientificus 39: 541, 1976.
- Marchoux, Pallois, Gebré-Sélassié, Caranta, Legnani & Dogimont, Annales du Tabac, Seita 2-27: 25, 1995.
- Marco & Cohen, Phytopathology 69: 1259, 1979.
- Marie-Jeanne Tordo, Chachulska, Fakhfakh, Le Romancer, Robaglia & Astier-Manifacier, Journal of General Virology 76: 939, 1995.
- Marte & Belleza, Phytopathologia Mediteranea 27: 169, 1988.
- Marte, Belleza & Polverari, Annals of Applied Biology 118: 309, 1991.
- Martelli, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 357, ed. R. G. Milne, New York: Plenum Press, 1988.
- Masuta, Nishimura, Morishita & Hataya, Phytopathology 89: 118, 1999.
- Matousek, Ptacek, Dedic & Schubert, Acta Virologica 44: 40, 2000.
- Mayee & Sarkar, Journal of Ulstrastructure Research 81: 124, 1982.
- McDonald & Bancroft, Journal of General Virology 35: 251, 1977.
- McDonald & Hiebert, Journal of Ultrastructure Research 48: 138, 1974.
- McDonald & Kristjanson, Plant Disease 77: 87, 1993.
- McDonald & Singh, American Potato Journal 73: 309, 1996a.
- McDonald & Singh, American Potato Journal 73: 317, 1996b.
- McDonald & Singh, Canadian Journal of Plant Pathology 19: 138, 1997.
- McDonald, Beveridge & Bancroft, Virology 69: 327, 1976.
- McDonald, Kristjansson, Singh, Ellis & McNab, American Potato Journal 71: 175, 1994.
- McDonald, Brandle, Gleddie, Hermans & Kermali, Canadian Journal of Plant Science 77: 167, 1997a.
- McDonald, Wong, Henning & Tao, Canadian Journal of Plant Pathology 19: 138, 1997b.
- McKern, Shukla, Barnett, Vetten, Dijkstra, Whittaker & Ward, Intervirology 33: 121, 1992.
- Merlet, Le Hingrat, Ellisèche, Crouau & Langlade, in La Pomme de Terre, p. 415, ed. P. Rousselle, Y. Robert & J.C. Crosnier, Paris: INRA éditions, 1996.
- Mestre, Brigneti & Baulcombe, The Plant Journal 23: 653, 2000.
- Mestre, Brigneti, Durrant & Baulcombe, The Plant Journal 36: 755, 2003.
- Miki & Oshima, Journal of General Virology 15: 179, 1972.
- Milne, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 333, ed. R. G. Milne, New York: Plenum Press, 1988.
- Mink, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 337, ed. R. G. Milne, New York: Plenum Press, 1988.
- Moghal & Francki, Virology 73: 350, 1976.
- Moghal & Francki, Virology 112: 210, 1981.
- Moravec, Cerovska & Boonham, Journal of Virological Methods 109: 63, 2003.
- Moury, Morel, Johansen & Jacquemond, Journal of General Virology 83: 2563, 2002.
- Moury, Morel, Johansen, Guilbaud, Souche, Ayme, Caranta, Palloix & Jacquemond, Molecular Plant-Microbe Interactions 17: 322, 2004.
- Munoz, American Potato Journal 52: 107, 1975.
- Naderi & Berger, Physiological and Molecular Plant Pathology 50: 67, 1997.
- Nelson & Wheeler, Phytopathology 68: 979, 1978.
- Nicolaisen, Rasmussen, Husted & Nielsen, Plant Pathology 50: 124, 2001.
- Nie & Singh, Journal of Virological Methods 86: 179, 2000.
- Nie & Singh, Journal of Virological Methods 103: 145, 2002a.
- Nie & Singh, Journal of Virological Methods 104: 41, 2002b.
- Nie & Singh, Journal of Virological Methods 113 : 69, 2003a.
- Nie & Singh, Virus Genes 26: 39, 2003b.
- Noguchi, Tajima, Yamamoto, Ohno & Kubo, Molecular Genetics and Genomics 262: 822, 1999.
- Ohshima, Inoue, Ishikawa, Shikata & Hagita, Annals of the Phytopathological Society of Japan 56: 508, 1990.
- Ohshima, Nakaya, Inoue, Hataya, Hayashi & Shikata, Journal of Virological Methods 40: 265, 1992.
- Ohshima, Sako, Hiraishi, Nakagawa, Matsuo, Ogawa, Shikata & Sako, Plant Disease 84: 1109, 2000.
- Okamoto, Nielsen, Albrechtsen & Borkhardt, Potato Research 39: 271, 1996.
- Orlob, Virology 35: 121, 1968.
- Oosterveld, in Viruses of potatoes and seed-potato production, 2nd edition, p. 204, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987.
- Ounouna, Kerlan, Lafaye, Loukili & El Gaaied, Plant Pathology 51: 487, 2002.
- Parella, Ruffel, Moretti, Morel, Palloix & Caranta, Theoretical and Applied Genetics 105: 855, 2002.
- Pehu, Mäki-Valkama, Valkonen, Koivu, Lehto & Pehu, American Potato Journal 72: 523, 1995.
- Pérennec, Cryptogamie-Mycologie 3: 377, 1982.
- Peters, in Viruses of potatoes and seed-potato production, 2nd edition, p.69, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987a.
- Peters, in Viruses of potatoes and seed-potato production, 2nd edition, p. 126, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987b.
- Peters, in Viruses of potatoes and seed-potato production, 2nd edition, p.171, ed. by J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987c.
- Piron, Netherlands Journal of Plant Pathology 92: 223, 1986.
- Pirone, Phytopathology 71: 922, 1981.
- Pirone, Seminars in Virology 2: 81, 1991.
- Pirone & Thornbury, Phytopathology 73: 872, 1983.
- Pirone & Blanc, Annual Review of Phytopathology 34: 227, 1996.
- Płochocka, Wełnicki, Zielenkiewicz & Ostoja-Zagόrski, Proceedings of the National Academy of Sciences, USA 93: 12150, 1996.
- Pontis & Feldman, Plant Disease Reporter 47: 22, 1963.
- Powell, Annals of Applied Biology 119: 313, 1991.
- Powell, Entomologia experimentalis et applicata 66: 255, 1993.
- Powell, Harrington & Spiller, Entomologia experimentalis et applicata 62: 293, 1992.
- Powell, Pirone & Hardie, European Journal of Plant Pathology 101: 411, 1995.
- Procenko, Phytopathologische Zeitschrift 68: 41, 1970.
- Proeseler & Weidling, Archiv für Phytopathologie und Pflanzenschutz 11: 335, 1975.
- Purcifull & Batchelor, Florida Agricultural Experiment Station Technical Bulletin 788, 1977.
- Purcifull & Gooding, Phytopathology 60: 1036, 1970.
- Purcifull, Hiebert & McDonald, Virology 55: 275, 1973.
- Purcifull, Zitter & Hiebert, Phytopathology 65: 559, 1975a .
- Purcifull, Christie & Batchelor, Phytopathology 65: 1202, 1975b.
- Quéméneur, La Pomme de Terre Francaise 371: 5, 1976.
- Racman, McGeachy, Reavy, Strukelj, Zel & Barker, Annals of Applied Biology 139: 269, 2001.
- Ramallo, d'Elba de Diaz & Whet, Revista Agronomica del Noroeste Argentino 15: 175, 1978.
- Ramirez, Gamez & Gonzalez, Phytopathology 54: 500, 1964.
- Revers, Legall, Candresse, Le Romancer & Dunez, Journal of General Virology 77: 1953, 1996.
- Richardson, Plant Pathology 7: 133, 1958.
- Robaglia, Durand-Tardif, Tronchet, Boudazin, Astier-Manifacier & Casse-Delbartet, Journal of General Virology 70: 935, 1989.
- Romero, Blanco-Urgoiti, Soto, Fereres & Ponz, Virus Research 79: 71, 2001.
- Rose & Hubbard, Annals of Applied Biology 109: 317, 1986.
- Rose, McCarra & Mitchell, Plant Pathology 36: 95, 1987.
- Rosner & Maslenin, Potato Research 42: 215, 1999a.
- Rosner & Maslenin, Journal of Phytopathology 147: 661, 1999b.
- Rosner & Raccah, Virus Genes 1: 255, 1988.
- Ross, Potato Breeding: Problems and perspectives, Berlin & Hamburg: Verlag Paul Parey, 1986.
- Rouis, Traincard, Gargouri, Dartevelle, Jeannequin, Mazié & Ayadi, Archives of Virology 146: 1297, 2001.
- Rozendaal, Van Binsbergen, Anema, Van Slogteren & Burt, Potato Research 14: 241, 1971.
- Ruffel, Dussault, Palloix, Moury, Bendahmane, Robaglia & Caranta, The Plant Journal 32: 1067, 2002.
- Ryden, Brishammar & Sigvald, Potato Research 26: 229, 1983.
- Ryšlavá, Muller, Semarádová, Synková & Čeřovská, Photosynthetica 41: 357, 2003.
- Sakimura, Phytopathology 43: 217, 1953.
- Salazar & Mink, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 343, ed. R. G. Milne, New York: Plenum Press, 1988.
- Salazar, Bartolini, & Flores, Fitopatologia 35: 87, 2000.
- Sanz, Cambra, Perez de San Roman, Miguet, Cortes, Gorris & Vela, Potato Research 33: 365, 1990.
- Sassaya, Torrance, Cowan & Ziegler, Journal of General Virology 81: 1115, 2000.
- Schepers, Abstracts, 9th Triennial Conference of the European Association for Potato Research, Interlaken: 204, 1984.
- Schmelzer, Bartels & Klinkowski, Phytopathologische Zeitschrift 40: 52, 1960.
- Schubert, Matousek & Mattern, Virus Research 100: 41, 2004.
- Schulz, Plant Disease Reporter 47: 594, 1963.
- Shands, American Potato Journal 54: 179, 1977.
- Shepard, Secor & Purcifull, Virology 58: 464, 1974.
- Shepherd, Fulton & Wakeman, Phytopathology 59: 219, 1969.
- Shukla & Ward, Archives of Virology 106: 171, 1989.
- Shukla, Inglis, McKern & Gough, Virology 152: 118, 1986.
- Shukla, McKern, Gough, Tracy & Lehto, Journal of General Virology 69: 493, 1988a.
- Shukla, Strike, Tracy, Gough & Ward, Journal of General Virology 69: 1497, 1988b.
- Shukla, Thomas, McKern, Tracy & Ward, Archives of Virology 102: 207, 1988c.
- Shukla, Jilka, Tosic & Ford, Journal of General Virology 70: 13, 1989.
- Shukla, Frenkel & Ward, Canadian Journal of Plant Pathology 13: 178, 1991.
- Shukla, Lauricella & Ward, in Potyvirus Taxonomy, p.57, ed. O.W. Barnett, Wien & New-York: Springer, 1992.
- Shukla, Ward & Brunt, The Potyviridae, Wallingford: CAB International, 1994.
- Shukla, Ward, Brunt & Berger, AAB Descriptions of Plant Viruses 366: 1998.
- Sigvald, Potato Research 27: 285, 1984.
- Sigvald, Potato Research 28: 135, 1985.
- Sigvald, Netherlands Journal of Plant Pathology 98 Supplement 2: 55, 1992.
- Silberschmidt, American Potato Journal 37: 151, 1960.
- Simons, Phytopathology 46: 53, 1956.
- Šindeláŕ, Šindeláŕová, Čeŕovská & Hanušova, Biologia Plantarum 32: 119, 1990.
- Šindelářová, Šindeláŕ & Burketová, Physiological and Molecular Plant Pathology 57: 191, 2000.
- Singh, Canadian Plant Disease Survey 72: 113, 1992.
- Singh, Journal of Virological Methods 74: 125, 1998.
- Singh, Genome 42: 592, 1999.
- Singh & Barker, Potato Research 34: 451, 1991.
- Singh & Singh, Journal of Virological Methods 52: 133, 1995.
- Singh & Singh, Canadian Journal of Plant Pathology 18: 209, 1996.
- Singh, Boucher, Somerville & Dhar, Canadian Journal of Plant Pathology 15: 293, 1993.
- Singh, Singh & Somerville, American Potato Journal 71: 567, 1994.
- Singh, Kurz & Boiteau, Journal of Virological Methods 59: 189, 1996.
- Singh, Singh & McDonald, Canadian Journal of Plant Pathology 20: 227, 1998.
- Singh, Singh & Moore, American Journal of Potato Research 75: 61, 1999.
- Singh, McLaren, Nie & Singh, Plant Disease 87: 679, 2003.
- Skofenko, Kushnirenko & Kolomiyetz, Abstracts of the 3rd International Congress of Virology, Madrid: W16, 1975.
- Skofenko, Kushnirenko & Kolomietz, Mikrobiologichnii Zhurnal 39: 358, 1977.
- Smith, Proceedings of the Royal Society of London, Series B 109: 251, 1931.
- Solomon-Blackburn & Barker, Heredity 86: 8, 2001a.
- Solomon-Blackburn & Barker, Heredity 86: 17, 2001b.
- Spetz, Taboada, Darwich, Ramsell, Salazar & Valkonen, Journal of General Virology 84: 2565, 2003.
- Stace-Smith & Tremaine, Phytopathology 60: 1785, 1970.
- Stobbs, Poysa & Van Schagen, Canadian Journal of Plant Pathology 16: 43, 1994.
- Sturz, Diamond & Stewart, Canadian Journal of Plant Pathology 19: 145, 1997.
- Sudarsono, Woloshuk, Xiong, Hellmann, Wernsman, Weissinger & Lommel, Archives of Virology 132: 161, 1993.
- Sudarsono, Young, Woloshuk, Parry, Hellmann, Wernsman, Lommel & Weissinger, Phytopathology 85: 1493, 1995.
- Szemes, Klerks, van den Heuvel & Schoen, Journal of Virological Methods 100: 83, 2002.
- Tacke, Salamini & Rohde, Nature Biotechnology 14: 1597, 1996.
- Talley, Frances, Warren, Torrance & Jones, Plant Pathology 29: 77, 1980.
- Thole, Dalmay, Burgyan & Balázs, Gene 123: 149, 1993.
- Thomas, Australasian Plant Pathology 10: 61, 1981.
- Thomas & MacGrath, Australian Journal of Agricultural Research 39: 475, 1988.
- Thompson, Hoffmann & Prins, Potato Research 30: 219, 1987.
- Thornbury & Pirone, Virology 125: 487, 1983.
- Thornbury, Hellman, Rhoads & Pirone, Virology 144: 260, 1985.
- Thornbury, Patterson, Dessens & Pirone, Virology 178: 573, 1990.
- Todd, Proceedings of the 4th Conference on Virus Diseases, Braunschweig: 82, 1961.
- Tollin & Wilson in The Plant Viruses, vol.4, The filamentous plant viruses, p. 51, ed. R. G. Milne, New York: Plenum Press, 1988.
- Tomlinson, Annals of Applied Biology 110: 661, 1987.
- Torrance, Annals of Applied Biology 96: 45, 1980.
- Urcuqui-Inchima, Maia, Drugeon, Haenni & Bernardi, Journal of General Virology 80: 2809, 1999a.
- Urcuqui-Inchima, Walter, Drugeon, German-Retana, Haenni, Candresse, Bernardi & Le Gall, Virology 258: 95, 1999b.
- Valkonen, Plant Breeding 112: 1, 1994.
- Valkonen, Annals of Applied Biology 130: 91, 1997.
- Valkonen, Brigneti, Salazar, Pehu & Gibson, Annals of Applied Biology 120: 301, 1992.
- Valkonen, Slack, Plaisted & Watanabe, Plant Disease 78: 177, 1994.
- Valkonen, Rokka & Watanabe, Phytopathology 88: 1073, 1998.
- Vance, Moore, Turpen, Bracken & Hollowell, Virology 191: 19, 1992.
- Van der Vlugt & Goldbach, Transgenic Research 2: 109, 1993.
- Van der Vlugt, Allefs, De Haan & Goldbach, Journal of General Virology 70: 229, 1989.
- Van der Vlugt, Ruiter & Goldbach, Plant Molecular Biology 20: 631, 1992.
- Van der Vlugt, Prins & Goldbach, Virus Research 27: 185, 1993.
- Van der Zaag, in Viruses of potatoes and seed-potato production, 2nd edition, p. 146, ed. J.A. De Bokx & J.P.H. Van der Want, Wageningen: Pudoc, 1987.
- Van Harten, Potato Research 26: 1, 1983.
- Van Hoof, Netherlands Journal of Plant Pathology 83: 123, 1977.
- Van Hoof, Netherlands Journal of Plant Pathology 85: 31, 1979.
- Van Hoof, Netherlands Journal of Plant Pathology 86: 159, 1980.
- Van Regenmortel, in Serology and immunochemistry of plant viruses, p.36, ed. M.H.V. Van Regenmortel, New York, London: Academic Press, 1982.
- Vardi, Sela, Edelbaum, Livneh, Kuznetsova & Stram, Proceedings of the National Academy of Sciences, USA 90: 7513, 1993.
- Varma, in The Plant Viruses, vol.4, The filamentous plant viruses, p. 371, ed. R. G. Milne, New York: Plenum Press, 1988.
- Varma, Gibbs, Woods & Finch, Journal of General Virology 2: 107, 1968.
- Varveri, Phytoparasitica 28: 1, 2000.
- Veerisetty, Virology 84: 523, 1978.
- Veerisetty, Intervirology 11: 167, 1979.
- Vetten, Ehlers & Paul, Phytopathologische Zeitschrift 108: 41, 1983.
- Vidal, Cabrera, Andersson, Fredriksson & Valkonen, Molecular Plant-Microbe Interactions 15: 717, 2002.
- Vincente, Chagas & July, Fitopatologia Brasileira 4: 73, 1979.
- Vuento, Paananen, Vihinen-Ranta & Kurppa, Biochimica et Biophysica Acta 1162: 155, 1993.
- Walkey & Webb, Phytopathologische Zeitschrift 110: 319, 1984.
- Walkey, Lyons & Taylor, Plant Pathology 41: 462, 1992.
- Walsh, North, Barker & Boonham, Journal of Virological Methods 91: 167, 2001.
- Wang, Ammar, Thornbury, Lopez-Moya & Pirone, Journal of General Virology 77: 861, 1996.
- Watson, Annals of Applied Biology 44: 509, 1956.
- Watson & Roberts, Proceedings of the Royal Society of London, Series B 127: 543, 1939.
- Webb & Wilson, American Potato Journal 55: 15, 1978.
- Weidemann, Potato Research 31: 85, 1988a.
- Weidemann, Potato Research 31: 485, 1988b.
- Weidemann & Maiss, Journal of Plant Disease Protection 103: 337, 1996.
- Weilgunny & Singh, Journal of Virological Methods 71: 57, 1998.
- Weintraub, Ragetli & Lo, Journal of Ultrastructure Research 46: 131, 1974.
- Wenzl & Foschum, Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 80: 341, 1973.
- Wernsman, Bulletin Spécial Coresta, Coresta symposium, Hellas (Greece): 113, 1991.
- Yamamoto, Bulletin of the Leaf Tobacco Research Laboratory 2: 78, 1992.
- Yamasaki, Yamamoto, Nieda & Uhara, Bulletin of the Leaf Tobacco Research Laboratory 2: 191, 1992.
- Young, Woloshuk, Parry, Hellmann, Wernsman, Lommel & Weissinger, Phytopathology 85: 1493, 1995.
- Zhang, Valkonen & Watanabe, Breeding Science 53: 155, 2003.
- Zitter, Plant Disease Reporter 56: 586, 1972.


