Details of DPV and References
DPV NO: 421 April 2011
Family: Rhabdoviridae
Genus: Nucleorhabdovirus
Species: Eggplant mottled dwarf virus | Acronym: EMDV
This is a revised version of DPV 115
Eggplant mottled dwarf virus
Giovanni P. Martelli Department of Plant Protection and Applied Microbiology, University of Bari, Italy and Plant Virology Institute of the CNR, UOS Bari, Bari, Italy
Marcello Russo Plant Virology Institute of the CNR, UOS Bari, Bari, Italy
Luisa Rubino Plant Virology Institute of the CNR, UOS Bari, Bari, Italy
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Eggplant mottled dwarf virus (EMDV) was first detected in Italy in eggplants (Solanum melongena) showing symptoms from which its name is derived (Martelli, 1969). EMDV is an enveloped virus with bacilliform particles c. 220-232 x 66-72 nm in plant tissue sections or c. 225-234 x 90-98 nm in leaf dips or partially purified preparations. It causes diseases in a number of hosts of different botanical families and is transmitted by inoculation of sap, grafting, leafhoppers and through seeds. The virus is a member of the genus Nucleorhabdovirus and, together with five additional genera (Vesiculovirus, Lyssavirus, Ephemerovirus, Novirhadbovirus and Cytorhabdovirus) is classified in the family Rhabdoviridae, order Mononegavirales (Tordo et al., 2005).
Main Diseases
Natural EMDV infections result in mottling, dwarfing and poor fruit set of eggplant (Martelli, 1969; Martelli & Cirulli, 1969); yellowing and curling of potato leaves, dwarfing of the plants, and production of small tubers with internal browning (Danesh & Lockhart, 1989; Mavrič et al., 2006); severe leaf crinkling of cucumber (Lecoq, 1982; Roggero et al., 1995) and tobacco (Polverari et al., 1996); clearing to intense yellowing of the veins of tomato, pepper, pelargonium, China rose (Hibiscus rosa-sinensis), pittosporum (Pittosporum tobira), honeysuckle (Lonicera japonica), caper (Capparis spinosa), and Solanum nigrum (Plavsić-Banjac et al., 1976; Di Franco et al., 1979; Plavsić et al., 1984; Castellano & Martelli, 1987; Martelli & Cherif, 1987; Lockhart, 1987; Roggero et al., 1995; Katis et al., 2011), and severe mosaic of Solanum sodomaeum (El Maataoui et al., 1985).
Geographical Distribution
The virus is endemic in the Mediterranean region. Records are from Portugal (Adam et al., 1987), continental Spain and Canary islands (Plavsić et al., 1984; Aramburu et al., 2006), France (Lecoq, 1982), Italy (Martelli, 1969), continental Greece and Rhodes island (Plavsić et al., 1984; Katis et al., 2000; 2011), Morocco (El Maataoui et al., 1985), Algeria (Martelli & Hamadi, 1986), Tunisia (Cherif & Martelli, 1985; Martelli & Cherif, 1987), Libya (Plavsić et al., 1978), Israel (Martelli, 1990), Jordan (Al-Musa & Lockhart, 1990), and Turkey (Martelli et al, 1984). It also occurs in several eastern European and Near East countries, i.e. Slovenia (Mavrič et al., 2006), Croatia (Plavsić-Banjac et al., 1976), Bulgaria (Neshev & Lecoq, 1996; Kostova et al., 2001), Iran (Danesh & Lockhart, 1989), and Afghanistan (Martelli & Lal, 1985), as well as in Nigeria (Ladipo, 1977) and Japan (Kano et al., 1985).
Host Range and Symptomatology
The natural host range is diversified, comprising vegetable and field crops (eggplant, tomato, potato, pepper, tobacco), woody and herbaceous ornamentals (pittosporum, China rose, honeysuckle, pelargonium), and wild plants (caper, Solanum nigrum and S. sodomaeum). Symptomless hosts are alfalfa (Danesh et al., 1989), Withania somnifera and Euphorbia geniculata (Al-Musa & Lockhart, 1990). The virus is readily transmitted by inoculation of sap to a rather restricted range of experimenatal hosts in the families Solanaceae, Chenopodiaceae, Amaranthaceace and Tetragonaceae, with a prevalence of the former (Martelli & Rana, 1970; Ladipo, 1977; Di Franco et al., 1979; Rana & Di Franco 1979; El Maataoui et al., 1985; Kano et al., 1985; Martelli & Cherif, 1987; Katis et al., 2011).
-
Diagnostic species
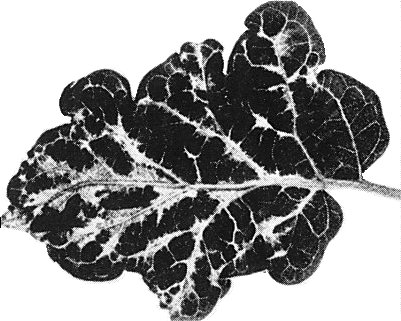
- Solanum melongena. Small chlorotic local lesions 2-3 weeks post inoculation followed by systemic mottling, severe vein clearing, leaf deformation and crinkling (Fig. 1).
- Nicotiana glutinosa. Chlorotic local lesions on inoculated leaves 10-15 days post inoculation, followed by systemic vein yellowing (Fig. 2), flecking of the leaves and stunting. Basal leaves wither and dry out.
- Nicotiana tabacum. Cvs Samsun, White Burley and Xanthi-nc react like N. glutinosa, but exhibit more obvious chlorotic local lesions (Fig. 3).
- Nicotiana rustica. Local and systemic symptoms similar to those shown by other Nicotiana species.
- Nicotiana glutinosa. Chlorotic local lesions on inoculated leaves 10-15 days post inoculation, followed by systemic vein yellowing (Fig. 2), flecking of the leaves and stunting. Basal leaves wither and dry out.
-
Propagation species
- S. melongena, N. glutinosa and N. tabacum are useful for maintaining cultures. N. rustica and N. glutinosa are a good source of virus for purification.
-
Assay species
- N. tabacum, and to lesser extent, N glutinosa, can be used for local lesion assays.
Strains
Biological variants
Differences in host range reactions were reported between a Greek virus isolate from cucumber and Italian isolates from cucumber and tobacco (Katis et al., 2011), a tomato isolate from Japan (Kano et al., 1985) and an eggplant isolate from Jordan (Al-Musa & Lockhart, 1990).
Serological variants
None apparently found (Adam et al., 1987; Katis et al., 2000; Kostova et al., 2001).
Molecular variants
The virus seems to be rather stable genetically. A slight molecular divergence was found between Greek mainland isolates and one from Rhodes island (Katis et al., 2011).
Transmission by Vectors
The virus is transmitted in nature by the leafhoppers Anaceratogallia laevis and An. ribauti (Della Giustina et al., 2000) and Agallia vorobjevi (Babaie & Izadpanah, 2003). Modality of transmission is undetermined but likely to be circulative, by analogy with that of other members of the genus (Peters, 1981). Experimental transmission by the aphids Myzus persicae, Aphis fabae, A. spiraecola, A. gossypii and Toxoptera aurantii and the planthopper Laodelfax striatellus was unsuccesful (Martelli & Russo, 1973; Rana & Di Franco, 1979; Kano et al., 1985; El Maataoui et al., 1985; Al-Musa & Lockhart, 1990).
Transmission through Seed
None found in Nicotiana rustica, N. glutinosa, N. debneyi, Solanum sodomaeum and S. nigrum (El Maataoui et al., 1985; Al-Musa & Lockhart, 1990).
Transmission by Grafting
The virus is readily transmitted by grafting from naturally infected hosts to plants of the same or related species (Corte, 1957; Martelli & Cirulli, 1969; Rana & Di Franco, 1979; Lecoq, 1982; Al-Musa & Lockhart, 1990).
Serology
The virus is a poor immunogen. Polyclonal antisera with titres up to 1:64 raised in rabbits immunized with purified virus preparations (El Maataoui et al., 1985; Adam et al., 1987) yield a single precipitin band in gel diffusion tests (El Maataoui et al., 1985; Castellano & Martelli, 1987; Martelli & Cherif, 1987; Lockhart, 1987; Polverari et al., 1996) and decorate virus particles from purified preparations and leaf dips (Martelli & Hamadi, 1986; Castellano & Martelli, 1987; Martelli & Cherif, 1987; Polverari et al., 1996; De Stradis et al., 2008; Katis et al., 2011). ELISA has been used for virus detection in weeds and field-grown hosts (Al-Musa & Lockhart, 1990; Mavrič et al., 2006; Aramburu et al, 2006; Pourrahim et al., 2007; Alfaro-Fernandez et al., 2011), and in the leafhopper A. vorobjevi (Babaie & Izadpanah, 2003). Virus isolates from different naturally infected hosts do not appear to differ serologically (Adam et al., 1987; Kostova et al., 2001; Katis et al., 2011).
Nucleic Acid Hybridization
RT-PCR and immunocapture RT-PCR using virus-specific primers designed on the viral polymerase and glycoprotein gene sequences, respectively, have been employed for virus detection in naturally infected hosts (Alfaro-Fernandez et al., 2011; Katis et al., 2011).
Relationships
The virus is serologically unrelated to the constricta and sanguinolenta strains of Potato yellow dwarf virus (PYDV) (El Maataoui et al., 1985), Broccoli necrotic yellows virus (BNYV) and Sonchus yellow net virus (SYNV) (Adam et al., 1987). The phylogenetic relationship with PYDV, Rice yellow stunt virus (RYSV) and Iranian maize mosaic virus (IMMV) is very distant, based on the percentage of glycoprotein gene identity at the amino acid level (Katis et al., 2011).
Stability in Sap
The virus is rather labile. Depending on the isolate, in N. glutinosa sap thermal inactivation point (10 min) ranges from 54 to 60°C, dilution end point from 10-3 and 10-6, and infectivity survives for 1 to 3 days (Martelli & Rana, 1970; Di Franco et al., 1979).
Purification
Method 1 (Russo & Martelli, 1973). Deribbed leaves of infected N. glutinosa or N. tabacum are chopped to small pieces and vacuum-infiltrated in an ice bath for 1 h with 3-4 volumes of a 0.1 M glycine, 0.01 M MgCl2 buffer, pH 8 before homogeneization. The slurry is adjusted to pH 7, centrifuged at 5,000 g for 30 min, treated with 10% Celite, then vacuum-filtered through a Celite pad. After centrifugation at 20,000 g for 1 h, pellets are resuspended in the glycine-magnesium chloride solution pH 7 and centrifuged in 10-30% sucrose density gradients at 22,000 rpm in a Spinco SW 25.1 rotor.
Method 2 (El Maataoui et al., 1985). Infected leaf tissue of N. rustica is blended in extraction buffer (0.2 M sodium citrate, pH 7.8, containing 0.01 M MgCl2 and 0.04 M Na2SO4). The homogenate is filtered through cheesecloth and centrifuged at 6,000 g for 10 min. The supernatant is vacuum-filtered through a Celite pad and centrifuged through a step gradient of 5 ml each of 30 and 60% sucrose in extraction buffer for 1 h at 25,000 rpm in a Beckman SW27 rotor. The material at the 30/60% interface is collected, diluted in the extraction buffer, and centrifuged for 25 min at 30,000 rpm in a Beckman type 35 rotor. Pellets are resuspended in 0.02 M sodium citrate containing 10% sucrose and clarified by a final low-speed centrifugation.
Method 3 (Adam et al., 1987). Infected N. rustica leaves are homogenized in extraction buffer (0.1 M glycine, 0.01 M MgCl2, pH 8.4), the slurry is strained through cheesecloth and the filtrate centrifuged for 10 min at 5,000 rpm. The supernatant is layered directly on a 15 ml cushion of 20% sucrose in 0.1 M glycine, 0.01 M MgCl2, pH 7.0 (VP) and centrifuged for 1 h at 23,000 rpm in a MSE 3x79 SW rotor. The pellet is dissolved in 0.2-0.5 ml VP and stored in aliquots in liquid nitrogen.
Properties of Particles
Sedimentation coefficient: virus sediments as a single component with a sedimentation coefficient (S20, w) of 1,040 S (Kano et al., 1985).
Particle Structure
Partially purified preparations fixed in glutaraldehyde prior to negative staining with phosphotungstate, contain bacilliform particles 220-230 x 90 nm in size, whereas in unfixed samples and leaf dips mounted in phosphotungstate, particles are mostly bullet-shaped (Fig. 7) and measure 60-190 x 80-90 nm (Russo & Martelli, 1973; Rana & Di Franco, 1979; El Maataoui et al., 1985). Unfixed particles mounted in uranyl formate and uranyl acetate are mostly bacilliform (Russo & Martelli, 1973; Rana & Di Franco, 1979; De Stradis et al., 2008). Virus particles exhibit surface projections c. 6 nm long, a helically arranged nucleocapsid with a pitch of 4.5 nm (Fig. 6) and an axial canal c. 18 nm across (Russo & Martelli, 1973; Di Franco et al., 1979; Martelli & Cherif, 1987). The nucleocapsid uncoils from particles exposed to sodium deoxycholate (Martelli & Hamadi, 1986; Martelli & Cherif; 1987). In cross section (Fig. 5), the particles show an outer envelope 10-12 nm thick which, depending on the fixing and staining procedure, may appear single- or double-layered. An electron-lucent space 5 nm across separates this external structure from an underlying ring c. 5.5 nm thick. The latter is, in turn, separated from an electron-lucent zone 4 to 4.5 nm wide from a central core c. 18 nm in diameter, exhibiting an electron-clear centre (Russo & Martelli, 1973). In tissue sections, 'immature' particles, i.e. those still attached to the nuclear envelope (Fig. 4) are bullet-shaped and have quite variable lengths, whereas 'mature' particles are bacilliform, have no apparent connection with the nuclear membrane and, generally, measure 220-232 x 66-72 nm (Martelli & Castellano, 1970; Martelli & Cherif, 1987; El Maataoui et al., 1985; Katis et al., 2011). Occasional bacilliform particles twice longer than normal (430-460 nm) seen in naturally infected eggplant tissues, are likely made up of two bullet-shaped nucleocapsids contained within the same envelope (Martelli & Castellano, 1970). Still longer rigid or slightly flexuous particles, reaching 1.5 µm in length, are found in naturally infected P. tobira plants and in N. benthamiana and N. glutinosa inoculated with the viral isolate from this host (Rana & Di Franco, 1979; Di Franco et al., 1980).
Particle Composition
Nucleic acid: Viral nucleic acid is an RNA consisting of a monopartite, single-stranded, linear, negative-sense molecule by analogy with the nucleic acid of other nucleorhabdoviruses (Peters, 1981).
Proteins: Virions contain five structural proteins with the following molecular weights (x103): <100 (L); 81-83 (G); 56-61 (N); 27-32 (M1) and 21-25 (M2) (Dale & Peters, 1981; Adam et al., 1987).
Lipids: The outer particle envelope contains lipids, as suggested by its reaction to differential electron microscope staining techniques (Martelli & Castellano, 1970; Russo & Martelli, 1973).
Genome Properties
Partial sequences of the G gene (246 nucleotides) of Greek virus isolates from five different hosts (Katis et al., 2011, AM22317-AM22321) and the polymerase gene (983 nt) of a Greek eggplant isolate (K.E. Efthimiou, unpublished information, AM922322) and a Spanish pittosporum isolate (376 nt) (Alfaro-Fernandez et al., 2011, HM636019) have been determined. The Greek EMDV G gene sequence has an identity at the amino acid level of 43, 34 and 31%, respectively with the comparable protein fragments of PYDV, RYSV and IMMV (Katis et al., 2011).
Relations with Cells and Tissues
The general structure of infected cells is fairly well preserved, even when the plants show severe symptoms. Chloroplasts and mitochondria exhibit only minor structural modifications but nuclei are severely affected, showing uniformly granular nucleoplasm and nucleoli of smaller size which are less dense than normal (Martelli, 1969; Martelli & Castellano, 1970; Rana & Di Franco, 1979). Infected nuclei are also mishapen and pick up cytochemical stains for DNA and histones differently from those of healthy cells. These changes originate from a depletion of chromatin after the nuclear involvement in virus multiplication (Russo & Martelli, 1975). Virus particles are present in leaf parenchyma tissues, in all parts of the flower, except stamens, and in the fruit pericarp of infected eggplants. They are also found in phloem and xylem parenchyma cells, but not in mature sieve and tracheary elements (Martelli, 1969; Martelli & Castellano, 1970; Russo & Martelli, 1972; Di Franco, 1985). Virus particles develop by budding at the nuclear envelope, with the nucleocapsid either oriented perpendicularly to the nucleus (Fig. 4) or, more rarely, parallel to the nuclear membrane. Particles accumulate, often in great numbers, in the perinuclear space within dilations of the nuclear envelope. They can also be present in the ground cytoplasm within an extended network of membranous cisternae connected to the nuclear membrane (Martelli, 1969; Martelli & Castellano, 1970; Russo & Martelli, 1972; El Maataoui et al., 1985; Martelli & Cherif, 1987; Castellano & Martelli, 1987; Polverari et al., 1996; Katis et al., 2011). Nicotiana and P. tobira cells infected with the pittosporum isolate react by forming cell wall protrusions that trap the unusually long virions that characterize this isolate.
Ecology and Control
The wide geographical distribution, the natural host range, that comprises major solanaceous crops, and the severity of the diseases induced, make EMDV a potentially serious pathogen. However, the incidence of infections is generally low. For instance, infections of eggplant rarely exceed 2% in southern Italy (Martelli & Cirulli, 1969), are less than 10% on tomato in Nigeria (Ladipo, 1977), less than 5% on cucumber in France (Lecoq, 1982) and 0.5 to 1.5% in Greece (Katis et al., 2000), 2% on tobacco in Italy (Polverari et al., 1996) and 5% on potato in Iran (Pourrahim et al., 2007). Higher, but still moderate infection rates were recorded on eggplant in Algeria (up to 20%) (Martelli & Hamadi, 1986) and Jordan (17%) (Al-Musa & Lockhart, 1990). Moreover, infections to ornamentals like pittosporum, China rose and honeysuckle are occasional (Corte, 1957; Plavsić et al., 1978; Martelli & Lal, 1985; Martelli & Cherif, 1987), or reach at the most 4%, as registered in Spain on pittosporum (Alfaro-Fernandez et al., 2011). This may depend on the efficiency of the vectors, which seems to vary with species. The rate of experimental virus transmission to cucumber by Anaceratogallia ribauti was 5%, 35% by An. laevis (Della Giustina et al., 2000), and 77% to eggplant by Agallia vorobjevi (Babaie & Izadpanah, 2003). Experimental transmission levels by An. laevis and A. vorobjevi are much higher than those registered in nature. This may indicate that, in the field, these vectors move from infected weed hosts, i.e. virus reservoirs (El Maataoui et al.,1985) on which they thrive, to crop plants, without colonizing and using them for further spread at a site. The fact that infected crop plants are located mostly at the margins of the plots (Martelli & Cirulli, 1969; Cherif & Martelli, 1985; Martelli & Hamadi, 1986; Polverari et al., 1996, Katis et al., 2000) seem to support this suggestion. The virus spreads also with infected propagating material, as shown by the interception in an Italian supermarket of infected potted plants of China rose imported from abroad (De Stradis et al., 2008).
Figures

Cross section of the periphery of a nucleus (N) showing virus particles budding at the nuclear envelope into spaces between the inner (il) and outer (ol) lamella of the membrane. Note the nuclear pore (np). Bar represents 200 nm.
References list for DPV: Eggplant mottled dwarf virus (421)
- Adam, Chagas & Lesemann, Journal of Phytopathology 120: 31, 1987.
- Alfaro-Fernandez, Cordoba-Selles, Tornos, Cebrian & Font, Plant Disease 95: 75, 2011.
- Al-Musa & Lockhart, Journal of Phytopathology 128: 283, 1990.
- Aramburu, Galipenso, Tornos & Matas, Plant Pathology 55: 565, 2006.
- Babaie & Izadpanah, Journal of Phytopathology 151: 679, 2003.
- Castellano & Martelli, Phytopathologia Mediterranea 26: 46, 1987.
- Cherif & Martelli, FAO Plant Protection Bulletin 33: 166, 1985.
- Corte, Rivista della Ortoflorofrutticoltura Italiana 41:197, 1957.
- Dale & Peters, Intervirology 16: 86, 1981.
- Danesh & Lockhart, Plant Disease 73: 856, 1989.
- Danesh, Bahar, Ahoonmanesh & Ghorbali, Proceedings of the 9th Plant Protection Congress of Iran, Mashad: 159, 1989.
- Della Giustina, Javory, Bansept, Morel, Balasse, Goussard & Passard, PHM-Revue Horticole 420: 40, 2000
- De Stradis, Parrella, Vovlas & Ragozzino, Journal of Plant Pathology 90: 359, 2008.
- Di Franco, Phytopathologia Mediterranea 24: 225, 1985.
- Di Franco, Russo & Martelli, Phytopathologia Mediterranea 18: 41, 1979.
- Di Franco, Russo & Martelli, Journal of General Virology 49: 221, 1980.
- El Maataoui, Lockhart & Lesemann, Phytopathology 75: 109, 1985.
- Kano, Namba, Yamashita, Doi & Yora, Annals of the Phytopathological Society of Japan 51: 602, 1985.
- Katis, Chatzivassilou, Avgelis, Manoussopoulos & Lecoq, Phytopathologia Mediterranea 39: 318, 2000.
- Katis, Chatzivassilou, Clay, Maliogka, Pappi, Efthimiou, Dovas & Avgelis, Journal of Plant Pathology 92, 2011 (in press)
- Kostova, Masenga, Milne & Lisa, Plant Disease 50: 804, 2001.
- Ladipo J.L., Plant Disease Reporter 61: 958, 1977.
- Lecoq, PHM-Revue Horticole 223: 15, 1982.
- Lockhart, Plant Disease 71: 731, 1987.
- Martelli, Journal of General Virology 5: 319, 1969.
- Martelli, Giornale di Agricoltura 100 (25): 45, 1990
- Martelli & Castellano, Phytopathologia Mediterranea 9: 39, 1970.
- Martelli & Cherif, Journal of Phytopathology 119: 32, 1987.
- Martelli & Cirulli, Annales de Phytopathologie Numero hors série: 392, 1969.
- Martelli & Hamadi, Plant Pathology 35: 595, 1986.
- Martelli & Lal, Phytopathologia Mediterranea 24: 228, 1985.
- Martelli & Rana, Phytopathologia Mediterranea 9: 3, 1970.
- Martelli & Russo, CMI/AAB Descriptions of Plant Viruses 115, 1973.
- Martelli, Yilmaz & Baloglou, Phytopathologia Mediterranea 23: 9, 1984
- Mavrič, Tusek Znidarič, Virsček Marn, Dolničar, Mehle, Lesemann & Ravnikar, Plant Pathology 55: 566, 2006.
- Neshev & Lecoq, Rastitelna Zashtita 9: 25, 1996.
- Peters, CMI/AAB Descriptions of Plant Viruses 244, 1981.
- Plavsić, Miličić & Erić, Phytopathologische Zeitschrift 91: 57, 1978.
- Plavsić, Erić & Miličić, Phytopathologia Mediterranea 23: 49, 1984.
- Plavsić-Banjac, Miličić & Erić, Phytopathologische Zeitschrift 86: 225, 1976.
- Polverari, Castellano & Marte, Journal of Phytopathology 144: 25, 1996.
- Pourrahim, Farzadfar, Golnaraghi & Ahoonmanesh, Plant Disease 91: 609, 2007.
- Rana & Di Franco, Phytopathologia Mediterranea 18: 48, 1979.
- Roggero, Milne, Masenga, Ogliara & Stravato, Plant Disease 79: 321, 1995.
- Russo & Martelli, Phytopathologia Mediterranea 11: 136, 1972.
- Russo & Martelli, Virology 52: 30, 1973.
- Russo & Martelli, Phytopathologische Zeitschrift 83: 97, 1975.
- Tordo, Benmansour, Calisher, Dietzgen, Fang, Jackson, Kurath, Nadin-Davies, Tesh & Walker, Virus Taxonomy. 8th Report of ICTV, p. 623, San Diego, CA, USA: Elsevier-Academic Press, 2005.