Details of DPV and References
DPV NO: 46 June 1971
Family: Bromoviridae
Genus: Alfamovirus
Species: Alfalfa mosaic virus | Acronym: AMV
There is a more recent description of this virus: DPV 229
Alfalfa mosaic virus
L. Bos Institute for Phytopathological Research, Wageningen, Netherlands
E. M. J. Jaspars Biochemical Dept, State University, Leiden, Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Weimer (1931,
1934).
Selected synonyms
- Lucerne mosaic virus (Rev. appl. Mycol. 24: 513)
- Alfalfa virus 1 and 2 (Rev. appl. Mycol. 13: 488)
- Medicago virus 2 (Smith, 1937)
- Marmor medicaginis (Rev. appl. Mycol. 28: 514)
- Alfalfa virus 1 and 2 (Rev. appl. Mycol. 13: 488)
-
An RNA-containing virus with bacilliform particles of three different lengths, the longest c. 60 nm. Readily transmissible by sap inoculation and in the non-persistent manner by aphids to a very wide range of host plants. Common in most countries.
Main Diseases
Causes mosaic in lucerne (Fig. 1), calico and tuber necrosis in potato (Fig. 3), various symptoms in tobacco (Fig. 5, Fig. 6, Fig. 7) and garden lupin, mosaic in chilli pepper, severe necrosis in tomato, mosaic in Malva parviflora, Daphne and Viburnum opulus, yellow fleck in Caryopteris incana, white mottle in Philadelphus sp., and is one of the causes of mosaic in red and white clover, mosaic or calico in celery and lettuce, and necrotic streak in pea; occurs naturally in many other herbaceous and some woody species (46 spp. in 12 families).
Geographical Distribution
World-wide.
Host Range and Symptomatology
Infects over 305 spp. in 47 dicotyledonous families (Hull, 1969). Sap transmission is easy between most hosts but may be difficult from lucerne. Symptoms greatly depend on virus strain, host variety and environmental conditions, and may be masked; recovery frequent. In many species mottle or mosaic is bright yellow (calico); severe necrosis may also occur. In lucerne and clover, yield is often reduced and predisposition to drought and winter injury increased.
-
Diagnostic species
- Phaseolus vulgaris
(French bean). Strains most commonly found in lucerne give necrotic (Fig. 4), sometimes spreading, lesions in most varieties; other strains produce chlorotic local lesions or none at all but give systemic mild mottle, vein necrosis and leaf distortion. - Vigna sinensis (cowpea). The commonest strains produce necrotic local
lesions; others infect
systemically.
- Vicia faba (broad bean). Most strains give black necrotic local lesions, sometimes followed by a mild mottle, but more often by stem necrosis and plant death.
- Pisum sativum (pea). In most varieties, necrotic local lesions and/or wilting of inoculated leaves with necrotic stem streaking and death of the plant.
- Chenopodium amaranticolor and C. quinoa. Local lesions; characteristic systemic chlorotic and necrotic flecking, which distinguishes alfalfa mosaic virus from most strains of cucumber mosaic virus.
- Nicotiana tabacum (tobacco). Necrotic or chlorotic local lesions (Fig. 5). Some strains give no local reaction. Systemic symptoms are mild mottle, bright chlorotic vein-banding, coalescing ringspots (Fig. 6), and, rarely, deformation (Fig. 7). Enations occur with certain strains. Plants often recover.
- Vicia faba (broad bean). Most strains give black necrotic local lesions, sometimes followed by a mild mottle, but more often by stem necrosis and plant death.
-
Propagation species
- Nicotiana tabacum
and N. glutinosa are suitable for maintaining cultures. N. tabacum, especially cultivars hypersensitive to tobacco mosaic virus, e.g. Samsun NN, are good sources of virus for purification.Assay species
- Phaseolus vulgaris
(most cultivars) and Vigna sinensis for strains that produce local lesions; Chenopodium amaranticolor and C. quinoa.
Strains
Numerous strains or variants with minor differences have been described.
Phaseolus vulgaris
and Vigna sinensis are mostly used for differentiation. Some more important
variants are:
Alfalfa yellow spot mosaic strain of
Zaumeyer (1963)
(Fig. 7);
AMV425 strain of Hagedorn
& Hanson (1963) (severe symptoms in red clover and Melilotus alba);
Chilli (Capsicum)
mosaic strain of
Berkeley (1947);
Potato calico strain of
Porter (1931);
Potato
tuber necrosis strain of
Oswald (1950);
AMV15/64 strain of Hull (1969).
Transmission by Vectors
Transmitted by at least 13 aphid spp. (Kennedy, Day & Eastop, 1962); no period of latency, transmission increased by starving the aphids before acquisition (Swenson, 1952), Myzus persicae can acquire the virus from purified preparations through Parafilm membranes (Pirone, 1964).
Transmission through Seed
Reported (up to 6%) in certain alfalfa varieties (Belli, 1962; Frosheiser, 1964; Zschau, 1964) and (1-5%) in chilli pepper (Sutic, 1959).
Transmission by Dodder
Occurs in at least five Cuscuta spp. (Schmelzer, 1956).
Serology
Moderately immunogenic, giving antibody titres of 1/1024 (Bancroft et al., 1960). AMY antisera contain three groups of antibodies, one specific for antigenic sites on the intact virus, the second specific for antigenic sites on the disaggregated virus protein and the third specific for both (Moed & Veldstra, 1968). The variously sized intact particles of the virus are serologically indistinguishable.
Relationships
No serological relationships to other viruses have been found. The bacilliform particles resemble those of a mushroom virus, and cacao swollen shoot and cacao mottle leaf viruses. Strains differing widely in pathogenicity and geographical origin were closely related serologically (Bancroft et al., 1960). Most strains, when tested in Phaseolus vulgaris, Nicotiana tabacum or N. glutinosa, cross protect against one another; however, lucerne isolates do not always completely protect against infection with isolates from Viburnum when tested in Petunia hybrida (Schmelzer, 1962).
Stability in Sap
The thermal inactivation point is usually between 60 and 65°C, but may range between 50 and 75°C, dilution end-point is around 10-3 but may approach 10-5, and longevity in vitro is usually 2-4 days. Infectivity seems best retained at pH 7.0-7.5 and when phosphate buffers (0.01 M to 0.5 M) are used for leaf extraction.
Purification
Modifications of Steere’s butanol/chloroform method are mostly used (Gillaspie & Bancroft, 1965) and yield up to 1.5 g virus per kg leaf tissue. Preparations sometimes contain ribosomal material. Alternatively, the virus may be precipitated with polyethylene glycol, M. Wt 20,000, in 0.2 M NaCl (Clark, 1968).
The virus components are separated by differential precipitation in 0.03 M MgSO4, and sucrose gradient centrifugation (Van Vloten-Doting, Kruseman & Jaspars, 1968), or by differential solubilization of a polyethylene glycol precipitate (Clark, 1968). Biological activity of purified components often decreases rapidly, but can be stabilized by ethylenediamino-tetraacetate (0.001 M).
Infective preparations of the RNA are best prepared by phenol extraction of the virus in 0.01 M phosphate buffer containing 1% sodium dodecyl sulphate or 2% sodium pyrophosphate. Infectivity of isolated RNA, assayed on Phaseolus vulgaris, is about 1% of that of intact virus. A convenient way to isolate the protein is to dissociate the virus in 0.5 M MgCl2; the RNA precipitates, and the supernatant fluid, after dialysing against 0.05 M acetate buffer, pH 5.5, contains the protein in dimer form.
Properties of Particles
Analytical ultracentrifugation of purified preparations reveals up to six components (Bancroft & Kaesberg, 1958; Hull, 1969), consisting of bacilliform particles of different lengths (Gibbs, Nixon & Woods, 1963). The three larger, obviously bacilliform, components (Fig. 2) are essential for infection and, in order of decreasing size, are named bottom (B), middle (M), and top b (Tb). The two or three spheroidal accessory top components (Ta, To, and Tz) are not infective, singly or together, and cannot replace the function of any of the three larger components of the infectious mixture (Van Vloten-Doting, Dingjan-Versteegh & Jaspars, 1970). Infections caused by a mixture of the functional components of two different virus strains give hybrid strains with properties of both parents. The relative amount of the different components depends on the virus strain and growing conditions.
Sedimentation coefficients (svedbergs) at infinite dilution: c. 99 (B), 89 (M), 75 (Tb), 68 (Ta), 60 (To) and 53 (Tz).
Molecular weights: c. 7.3 x 106 (B), 3.7 x 106 (Ta).
Electrophoretic mobility: -6.6 x 10-3 cm2sec-1 volt-1 at pH 7 and 0.1 ionic strength. The virus migrates as a single component. The RNA contributes to the surface charge (Bol & Veldstra, 1969). The virus is precipitated below pH 6. Above pH 4, precipitates retain infectivity for at least a few hours at 4°C.
A260/280: 1.7-1.8 (B, Ta and probably also M and Tb).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 5.2; may be lower for accessory components.
Buoyant density in CsCl after fixation with formaldehyde: 1.385 (B) and 1.382 (Ta).
The virus is sensitive to pancreatic ribonuclease (Pirone, 1962) and trypsin (Ross, 1941). With ribonuclease, particles lose RNA fragments and ultimately degrade to smaller structures (Bol & Veldstra, 1969). At high ionic strength (e.g. 1.5 M NaCl, pH 5.5) a partially reversible dissociation occurs (Bol & Kruseman, 1969). The virus has been partially reconstituted from isolated RNA and protein by Lebeurier, Wurtz & Hirth (1969) and Hull (1970).
Particle Structure
The components represent a series of six-sided elongated particles of different lengths, the cylindrical part of the particles consisting of an hexagonal lattice net (angle of prominent lattice vector with particle axis: 0°; lattice spacing: 4.8 nm. At either end is a half icosahedral structure bisected at right angles to a threefold axis. The subunits are possibly grouped in hexamer clusters in the cylindrical part and in 6 pentamer clusters at the ends. The particles in component Tz are probably icosahedral (by optical diffraction of electron micrographs) (Gibbs et al., 1963; Hull, Hills & Markham, 1969a). The lengths of the components (nm) in purified preparations (mounted in potassium phosphotungstate after fixation with formaldehyde) are about 58 (B), 49 (M), 38 (Tb), 29 (Ta) and 19 (Tz). In crude preparations particles over 1 µm in length have been observed. Diameter of all particles is about 18 nm. With uranyl acetate as negative stain, fixation is unnecessary.
Particle Composition
RNA: Single-stranded. About 18% of the weight of each of the three functional components. Approximate M. Wt of RNA: 1.3 x 106 (B), 1.1 x 106 (M), 0.9 x 106 (Tb). Ta probably contains two RNA molecules of M. Wt c. 0.3 x 106. M. Wt of RNAs from To and Tz unknown (Hull, Rees & Short, 1969b; Hull et al., 1969a; Bol, 1969). Sedimentation coefficients (svedbergs) at infinite dilution (solvent: 0.01 M Tris, 0.1 M KCl, pH 7): c. 26 (B) and 13 (Ta). Molar percentages of nucleotides: G23; A27; C21; U30 for B; and G24; A25; C23; U28 for Ta (Rauws, Jaspars & Veldstra, 1964). Nicolaieff, Pinck & Hirth (1969) isolated double-stranded RNA molecules 0.5 and 0.9 µm long directly from infected tobacco plants.
Protein: All components have the same coat protein. Hull et al. (1969b) reported that the protein subunit has a M. Wt of 32,600 (strain 15/64), but others have failed to confirm this. From hydrodynamic data and amino acid analyses a M. Wt of 24,500 has been derived for strain 425 whereas electrophoresis of the protein in sodium dodecyl sulphate/polyacrylamide gels suggests that it is about 27,500 (eight strains, including 425 and 15/64; Kruseman et al., 1971; J. M. Carpenter, pers. comm.). Amino acid compositions have been reported by Kelley & Kaesberg (1962), Hull et al. (1969b), Tremaine & Stace-Smith (1969) and Kruseman et. al. (1971).
Relations with Cells and Tissues
Transient, amorphous, granular inclusion bodies are seen by light microscopy in tobacco leaves (Desjardins, 1966). Electron microscopy of thin sections shows whorled aggregates and rafts of particles hexagonally packed side by side in the vacuoles and cytoplasm (Hull, 1969).
Notes
In host range, symptoms, vector relationships and many other properties alfalfa mosaic virus resembles cucumber mosaic virus. The latter, however, has spherical particles, produces local lesions in Phaseolus vulgaris only in winter, and most strains do not infect Chenopodium amaranticolor or C. quinoa systemically; moreover not all strains of alfalfa mosaic virus infect cucumber.
Figures
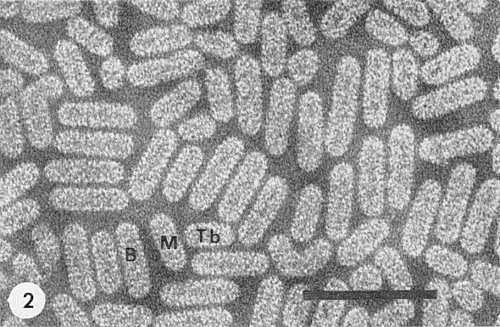
Electron micrograph of purified virus preparation, fixed with formaldehyde and mounted in phosphotungstate. B, bottom component; M, middle component; Tb, top component b. Spheroidal particles are the top components Ta, To or Tz. Bar represents 100 nm. (Courtesy Dr M. Verhoyen & Mr S. Henstra.)

Potato cv. Eersteling (Duke of York), with calico symptoms after inoculation with an isolate from white clover. (Photo I. P. O., Wageningen.)
References list for DPV: Alfalfa mosaic virus (46)
- Bancroft & Kaesberg, Nature, Lond. 181: 720, 1958.
- Bancroft, Moorhead, Tuite & Liu, Phytopathology 50: 34, 1960.
- Belli, Annali. Fac. Agr. Univ. Milano (1961)10, l5pp., 1962.
- Berkeley, Phytopathology 37: 781, 1947.
- Bol, Thesis University of Leiden, 1969.
- Bol & Kruseman, Virology 37: 485, 1969.
- Bol & Veldstra, Virology 37: 74, 1969.
- Clark, J. gen. Virol. 3: 427, 1968.
- Desjardins, Phytopathology 56: 875, 1966.
- Frosheiser, Phytopathology 54: 893, 1964.
- Gibbs, Nixon & Woods, Virology 19: 441, 1963.
- Gillaspie & Bancroft, Virology 27: 391, 1965.
- Hagedorn & Hanson, Phytopathology 53: 188, 1963.
- Hull, Adv. Virus Res. 15: 365, 1969.
- Hull, Virology 40: 34, 1970.
- Hull, Hills & Markham, Virology 37: 416, 1969a.
- Hull, Rees & Short, Virology 37: 404, 1969b.
- Kelley & Kaesberg, Biochim. biophys. Acta 61: 865, 1962.
- Kennedy, Day & Eastop, A conspectus of aphids as vectors of plant viruses, Commonwealth Institute of Entomology, London, 1962.
- Kruseman, Jaspars, Bol, Brederode & Veldstra, Biochemistry 10: 47, 1971.
- Lebeurier, Wurtz & Hirth, C.R. Acad. Sci. Paris 268D: 2002, 1969.
- Moed & Veldstra, Virology 36: 459, 1968.
- Nicolajeff, Pinck & Hirth, J. gen. Virol. 4: 283, 1969.
- Oswald, Phytopathology 40: 973, 1950.
- Pirone, Phytopathology 52: 747, 1962.
- Pirone, Virology 23: 107, 1964.
- Porter, Hilgardia 6: 277, 1931.
- Rauws, Jaspars & Veldstra, Virology 23: 283, 1964.
- Ross, Phytopathology 31: 394, 1941.
- Schmelzer, Phytopath. Z. 28: 1, 1956.
- Schmelzer, Phytopath. Z. 46: 17, 1962.
- Smith, Textbook of Plant Virus Diseases, Churchill, London, 1937.
- Sutic Phytopath. Z. 36: 84, 1959.
- Swenson,Phytopathology 42: 261, 1952.
- Tremaine & Stace-Smith, Phytopathology 59: 521, 1969.
- Van Vloten-Doting, Kruseman & Jaspars, Virology 34: 728, 1968.
- Van Vloten-Doting, Dingjan-Versteegh & Jaspars, Virology 40: 419, 1970.
- Weimer, Phytopathology 21: 122, 1931.
- Weimer, Phytopathology 24: 239, 1934.
- Zaumeyer, Phytopathology 53: 444, 1963.
- Zaumeyer & Wade, J. agric. Res. 51: 715, 1935.
- Zschau, NachrBl. dt. PflSchutzdienst, Berl. 18: 44, 1964.




