Details of DPV and References
DPV NO: 47 June 1971
Family: Secoviridae
Genus: Comovirus
Species: Cowpea mosaic virus | Acronym: CPMV
There is a more recent description of this virus: DPV 197
Cowpea mosaic virus
A. van Kammen Laboratorium voor Virologie, Wageningen, The Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Smith (1924) and
Dale (1949).
Synonym
- Cowpea yellow mosaic virus (Rev. appl. Mycol. 39: 204)
-
An RNA-containing virus with isometric particles about 28 nm in diameter. It has a limited host range, is transmitted by beetles and readily by inoculation of sap. Infected plants contain two kinds of nucleoprotein particles similar in size, but differing in RNA content; both are necessary for virus infectivity. Particles containing no RNA are also produced.
Main Diseases
Causes mosaic of Vigna spp. In Nigeria it greatly decreases the leaf area, flower production and yield of infected plants (Chant, 1960). Dale (1949) recorded natural infection of soya beans (Glycine max), mungo beans (Phaseolus mungo and P. aureus) and sunn hemp (Crotalaria juncea), but only when growing near to infected cowpeas.
Geographical Distribution
Reported from Nigeria, Trinidad, Surinam and USA.
Host Range and Symptomatology
Host range rather limited. Only dicotyledons have been described as hosts, but monocotyledons have not been tested systematically (Agrawal, 1964). The symptoms caused by the yellow and the severe strains (see Strains) differ greatly. Readily transmitted by inoculation of sap.
-
Diagnostic species
- Vigna unguiculata
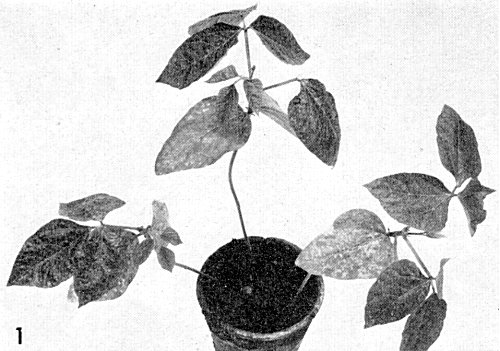
(cowpea). The yellow strain produces chlorotic lesions in the inoculated primary leaves, bright yellow mosaic and vein-yellowing in systemically infected trifoliate leaves (Fig. 1) and dark green spots and mosaic on the pods. The severe strain produces chlorotic and some necrotic spots in inoculated primary leaves and severe mosaic with vein-clearing, leaf distortion and necrosis in the trifoliate leaves. Necrosis of the stem just below the primary leaves and in the veins results in collapse of the plants (Fig. 2). - Phaseolus vulgaris cv. Beka (French bean). The yellow strain produces
yellow spots in inoculated leaves and mild systemic symptoms, some puckering of
the trifoliate leaves and local vein necrosis. The severe strain produces severe
systemic necrosis and leaf distortion, and plants usually collapse
(Fig. 6).
- Chenopodium amaranticolor. The yellow strain produces, in inoculated leaves, yellow local lesions (0.5-1 mm), later becoming necrotic, and in the upper leaves severe systemic mosaic, chlorotic spots, distortion and puckering (Fig. 3). The severe strain produces only local lesions and no systemic reaction (Fig. 4).
-
Propagation species
- Vigna unguiculata
or V. sinensis are good sources of virus for purification and for maintaining cultures.Assay species
- Phaseolus vulgaris
(cvs. Pinto and Scotia) and Chenopodium amaranticolor are suitable local lesion hosts.
Strains
Among isolates of the virus from Surinam, Trinidad and Nigeria, Agrawal (1964) distinguished ‘yellow’ and ‘severe’ strains by the type of symptoms they caused in V. unguiculata, C. amaranticolor and P. vulgaris cv. Beka. Little work has been done on the prevalence of different strains in nature. Bruening (1969) isolated a naturally occurring mutant of the yellow strain, which differs from the parent strain in the relative amount of RNA-free particles it produces. De Jager & Van Kammen (1970) treated the yellow strain with nitrous acid and obtained a mutant which gave no systemic reaction in Beka beans and produced a relatively large proportion of RNA-free particles.
Transmission by Vectors
Transmitted by various leaf beetles: Ceratoma ruficornis in Trinidad (Dale, 1953), Ootheca mutabilis in Nigeria (Chant, 1959) and Ceratoma trifurcata in USA (Smith, 1924). In Surinam the yellow strain was transmitted by Ceratoma variegata, but not by Diphaulaca sp. (probably D. meridae), whereas the severe strain was transmitted by Diphaulaca sp., Ceratoma variegata and Diabrotica sp. (probably D. laeta) (Van Hoof, 1963). C. ruficornis remained viruliferous for 14 days (Dale, 1953). Van Hoof (1963) reported that C. variegata remained viruliferous for only 4 days, but in his experiments the beetles soon died. Dale (1953) found that 30% of the beetles collected in the field were viruliferous and Van Hoof (1963) found 50% of the beetles used in an experiment able to transmit. Outbreaks of mosaic disease in cowpea crops are influenced by seasonal and climatic factors which determine the prevalence of vector beetles (Van Hoof, personal communication).
Transmission through Seed
An isolate from Trinidad was seed-transmitted in Vigna unguiculata, but not in Vigna sinensis (Dale, 1949; 1953). This virus is probably a representative of the severe strain (Agrawal, 1964).
Serology
The virus is strongly immunogenic. Standard methods give antisera with titres of 1/1024 in the Ouchterlony double diffusion test, but higher titres may be obtained. Virus preparations tested by the Ouchterlony method give a single band of precipitate, even though they contain more than one centrifugal or electrophoretic component.
Relationships
The yellow and severe strains are distantly serologically related: antiserum to each strain has a titre to homologous antigen at least sixteen times higher than that to heterologous antigen and absorption with heterologous antigen leaves the titre to homologous antigen virtually unchanged (Van Kammen, unpublished). Shepherd (1963) reported isolates from Arkansas and Trinidad to be related but not identical. Cross-absorption tests have not been reported, but cowpea mosaic virus is distantly related to bean pod mottle, red clover mottle (Agrawal, 1964; Shepherd, 1963), radish mosaic (Campbell, 1964), pea green mottle (Valenta & Gressnerova, 1966), squash mosaic and broad bean stain viruses (Gibbs, Giussani-Belli & Smith, 1968). These viruses form the cowpea mosaic virus group.
Stability in Sap
The thermal inactivation point (10 min) of the virus in Vigna sap is 65°-75°C. The dilution end-point is 10-4-10-5. In sap diluted 1/10 in 0.01 M phosphate buffer pH 7.0 and stored at room temperature, infectivity survived 3-5 days in vitro. Dale (1949) found crude sap remained infective over 20 days at room temperature.
Purification
Yields of virus may reach 2 g/kg leaf tissue from Vigna plants grown at 30°C in a growth chamber. In the chloroform-butanol method (Steere, 1956; Bruening & Agrawal, 1967), the frozen leaves are crushed and blended in twice their weight of buffer (0.02 M potassium acetate, 0.002 M EDTA, pH 5.8) at about 4°C. The mixture is filtered through gauze, mixed with an equal volume of a mixture (1:1) of chloroform and n-butanol, and stirred for 20 min. The phases are separated by centrifugation and the aqueous phase recovered. In an alternative method using polyethylene glycol (PEG) and NaCl (Hebert, 1963; Van Kammen, 1967), the frozen leaves are homogenized in 0.1 M phosphate buffer pH 7.0 (1 ml/g leaf). The homogenate is filtered and the extract centrifuged at 10,000 g for 15 min. To the supernatant fluid, PEG is added to a final concentration of 4% (w/v) and NaCl to give a concentration of 0.2 M. The mixture is stirred at room temperature to dissolve the PEG and NaCl and, after one hour, centrifuged at 10,000 g for 15 min; the pellets are resuspended in 0.01 M phosphate buffer, pH 7.0. The preparations obtained by these methods may then be further purified by differential centrifugation.
Properties of Particles
Purified preparations of the virus contain three centrifugal components, empty protein shells without RNA (T), and two nucleoprotein components (M and B) containing 24% and 33% RNA respectively. The separated nucleoprotein components are not infectious (Van Kammen, 1968), but mixtures of the M and B components are. The infectivity of a mixture depends upon the proportions of the two components and the concentration of the component present in the lowest amount. Mixtures of the M or B components of the yellow strain with the B or M components of mutants of the yellow strain are infectious but similar heterologous mixtures of components of the yellow and severe strains are not (Bruening, 1969; De Jager & Van Kammen, 1970). This again indicates that the yellow and severe strains differ considerably.
Sedimentation coefficients (s20,w) at infinite dilution (svedbergs): 58 (T), 95 (M), 115 (B). For the yellow strain the proportion of M:B is about 2:1, for the severe strain 10:1.
Molecular weight: about 6 x 106 (M) and 7.7 x 106 (B).
Isoelectric point: between pH 3.4 and 4.5.
Absorbances at 260 nm (1 mg/ml, 1 cm light path): 6.2 (M), 10.0 (B).
A260/A280: 0.69 (T), 1.57 (M), 1.67 (B).
Buoyant density in CsCl (g/ml): 1.30 (T), 1.41 (M); component B gives two bands of density 1.43 and 1.47. The two bands have the same sedimentation coefficient (Bruening, 1969; Van Kammen & Van Griensven, 1970).
Purified preparations of the virus contain two electrophoretic components, each of which contains all three centrifugal components (Semancik, 1966). Electrophoretic mobility (yellow strain): -4.0-4.25 x 10-5 and -2.6-2.8 x 10-5 cm2 sec-1 volt-1 in 0.1 M phosphate buffer pH 7.0. The mobilities are somewhat lower for the severe strain. The slower migrating form predominates in early infection and the faster one in late infection. The slower migrating form is reported to be converted into the faster migrating form in vivo. The conversion of the slower form into the faster form can be simulated in vitro by treating the preparation with a mixture of carboxypeptidases A and B or with chymotrypsin. This conversion is accompanied by an increase of about 85% in specific infectivity (Niblett & Semancik, 1969).
Particle Structure
Particles are isometric, c. 28 nm in diameter. The particles may be seen most clearly when negatively stained with phosphotungstate, but it is difficult to deduce their substructure from such pictures (Fig. 5) (Agrawal, 1964).
Particle Composition
RNA: The single RNA strands in components M and B have sedimentation coefficients (s20,w) of 26 S and 34 S and molecular weights of about 1.45 x 106 and 2.55 x 106 respectively. For the yellow strain their respective base compositions (molar percentages of nucleotides) are G 20.7; A 28.4; C 19.3; U 31.6 and G 22.9; A 28.5; C 17.2; U 31.4 (Van Kammen & Van Griensven, 1970).
Protein: Probably one type of protein with 159-165 amino acid residues. That of the faster electrophoretic form contains three amino acid residues (arg, glu, ile) fewer than that of the slower form (Niblett & Semancik, 1969).
Relations with Cells and Tissues
The virus particles occur scattered and in clusters throughout the cytoplasm. They do not form crystalline arrays (Fig. 7). Amorphous inclusion bodies frequently occur around or close to the nucleus in epidermal cells of infected Vigna leaves.
Notes
The yellow and severe strains of cowpea mosaic virus were both first isolated from Vigna spp.; however, they seem no more closely related to each other than to other members of the cowpea mosaic virus group (e.g. bean pod mottle virus, red clover mottle virus). Therefore it would perhaps be better to regard them not as strains but as separate members of the cowpea mosaic virus group.
Many other viruses have been isolated from Vigna spp. showing mosaic symptoms; the most notable of these are: cowpea aphid-borne mosaic virus (Lovisolo & Conti, 1966), a strain of bean southern mosaic virus (Shepherd & Fulton, 1962), cowpea chlorotic mottle virus (Kuhn, 1964) and a legume-infecting strain of tobacco mosaic virus (Lister & Thresh, 1955). Cowpea mosaic virus may be distinguished from these and other viruses of cowpea by the morphology of its particles and their behaviour in the ultracentrifuge, but serological tests seem to be the only sure way of identifying the virus.
Figures
References list for DPV: Cowpea mosaic virus (47)
- Agrawal, Meded. LandbwHoogesch. Wageningen 64-65: 54 pp., 1964.
- Bruening, Virology 37: 577, 1969.
- Bruening & Agrawal, Virology 32: 306, 1967.
- Campbell, Phytopathology 54: 1418, 1964.
- Chant, Ann. appl. Biol. 47: 565, 1959.
- Chant, Emp. J. exp. Agric. 28: 114, 1960.
- Dale, Ann. appl. Biol. 36: 327, 1949.
- Dale, Ann. appl. Biol. 40: 384, 1953.
- De Jager & Van Kammen, Virology 41: 281, 1970.
- Gibbs, Giussani-Belli & Smith, Ann. appl. Biol. 61: 99, 1968.
- Hebert, Phytopathology 53: 362, 1963.
- Kuhn, Phytopathology 54: 853, 1964.
- Lovisolo & Conti, Neth. J. Pl. Path. 72: 265, 1966.
- Lister & Thresh, Nature, Lond. 175: 1047, 1955.
- Niblett & Semancik, Virology 38: 685, 1969.
- Semancik, Virology 30: 689, 1966.
- Shepherd, Phytopathology 53: 865, 1963.
- Shepherd & Fulton, Phytopathology 52: 489, 1962.
- Smith, Science, N.Y. 60: 268, 1924.
- Steere, Phytopathology 46: 60, 1956.
- Valenta & Gressnerova, Acta virol., Prague 10: 182, 1966.
- Van Hoof, Surin. Landb. 11: 131, 1963.
- Van Kammen, Virology 31: 633, 1967.
- Van Kammen, Virology 34: 312, 1968.
- Van Kammen & Van Griensven, Virology 41: 274, 1970.