Details of DPV and References
DPV NO: 54 June 1971
Family: Potyviridae
Genus: Potyvirus
Species: Potato virus A | Acronym: PVA
Potato virus A
R. Bartels Biologische Bundesanstalt für Land- und Forstwirtschaft, Braunschweig, Germany
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Murphy & McKay (1932).
- Selected synonyms
- Marmor solani
(Rev. appl. Mycol. 18: 607) - Selected synonyms
- Potato mild mosaic virus (Rev. appl. Mycol. 18: 607)
- Potato virus P (Rev. appl. Mycol. 15: 391)
- Solanum virus 3 (Rev. appl. Mycol. 17: 52)
- Potato virus P (Rev. appl. Mycol. 15: 391)
- An RNA-containing virus with filamentous particles, normal length c. 730 nm. Only hosts known are in the Solanaceae. Transmitted by aphids in the non-persistent manner and by inoculation of sap. Widely distributed in potato-growing areas.
Main Diseases
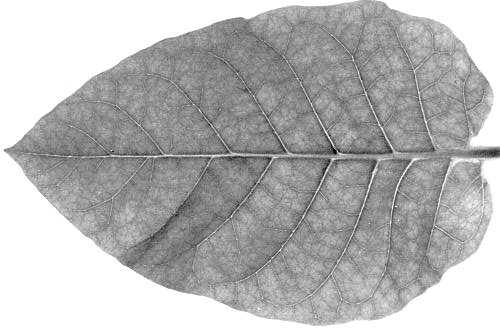
Leaves of infected potatoes may show a mild mosaic, roughness of the surface, waviness of the leaf margin (Fig. 1), or no symptoms at all depending on the variety and on the weather. Some hypersensitive varieties develop top necrosis. Potatoes infected with potato virus A in combination with potato virus X or potato virus Y show crinkle symptoms. Potato virus A decreases yield of infected potatoes by up to 40%.
Geographical Distribution
Widespread in most potato-growing countries.
Host Range and Symptomatology
Known hosts are limited to the Solanaceae. Sap-transmission without abrasive is usually difficult because of low virus concentration.
- Diagnostic species
- Nicotiana tabacum
(tobacco) cv. Samsun. Vein-clearing and diffuse mottle depending on strain and conditions of culture (Fig. 3). - N. tabacum (tobacco) cv. White Burley. Vein-clearing and dark green
vein-banding depending on strain and conditions of culture (Fig. 4).
- Nicandra physalodes. Slight vein-clearing and mottle to severe necrosis, rugosity and stunting, depending on virus strain.
- Lycopersicon pimpinellifolium. Systemic necrosis; plant usually dies (MacLachlan, Larson & Walker, 1953).
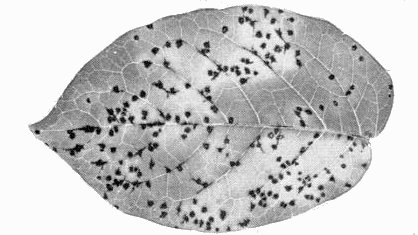
- Solanum demissum x S. tuberosum cv. Aquila (=A6 hybrid) and S. demissum ‘SdA’. Numerous local lesions (Fig. 2).
- Nicandra physalodes. Slight vein-clearing and mottle to severe necrosis, rugosity and stunting, depending on virus strain.
- Propagation species
- Nicotiana tabacum
cv. Samsun is a good source for purification. The maximum virus concentration in this host plant depends on the conditions of culture (Bartels, 1954).- Assay species
- Aquila (A-6) hybrid is a useful local lesion host. Detached inoculated leaves
kept in a humid
chamber at 24°C and illuminated by fluorescent lamps (1000 lux) give small
dark brown
dots with regular or star-shaped borders or intense black necrotic flecks (Fig. 2)
(Köhler,
1953; Bartels, 1970).
Solanum demissum ‘SdA’ gives necrotic local lesions
(Cockerham,
1958).
- For aphid transmission tests Nicandra physalodes is useful, being very susceptible to infection by Myzus persicae.
Strains
Strains differing in virulence towards potato and Nicandra physalodes may be classed into four groups: very mild, mild, moderately severe, severe (Calvert, 1960). Their virulence is correlated with their physical properties such as dilution end-point, thermal inactivation point and longevity in vitro (MacLachlan et al., 1953) and also with their behaviour in Nicotiana glutinosa (Schmelzer, 1959).
Transmission by Vectors
Transmissible by at least 7 species of aphids in the non-persistent manner, notably Aphis frangulae, A. nasturtii and Myzus persicae. Virus can be acquired by Myzus persicae in 20 sec, inoculated in 20 sec (MacLachlan et al., 1953), and retained for 20 min (Sylvester, 1954). No latent period. Myzus persicae can transmit potato aucuba mosaic virus when the source plants also contain potato virus A (Clinch, Loughnane & Murphy, 1936).
Transmission through Seed
None reported.
Transmission by Dodder
None reported.
Serology
Moderately immunogenic. Antisera with titres of 1/512 are usually obtained; maximum 1/4096 (Bartels, unpublished). With the slide-precipitin test virus can readily be detected in sap of infected tobacco, but not in sap from infected potato plants. The precipitates are flocculent (flagellar). No agar gel-diffusion tests are reported.
Relationships
Serologically related to potato Y (common and tobacco veinal necrosis strains), henbane mosaic and tobacco etch viruses, although the degree of relationship among these four viruses is difficult to assess, because reciprocal heterologous tests do not give the same results (Bartels, 1964; Fribourg & de Zoeten, 1970). These serological relationships, together with its particle morphology and other properties show that potato virus A is a member of the potato virus Y group of viruses (Brandes, 1964).
Stability in Sap
In Nicandra physalodes sap, different strains of virus lose infectivity after heating 10 min at 44-52°C, dilution 1/10 to 1/40, or storage for 12-18 hr at 18°C (MacLachlan et al., 1953). The virus did not survive lyophilization (Hollings & Stone, 1970).
Purification
Differential centrifugation of infective sap gives poor results and little or no virus is obtained. The following method, however, is successful, if done in the cold. Leaves are homogenized along with 0.2%(w/v) ascorbic acid and 0.2%(w/v) sodium sulphite, n-butanol is added dropwise to 8%(v/v) and the mixture centrifuged at low speed. The supernatant fluid is homogenized once or twice with an equal volume of CCl4 and the virus purified by differential centrifugation (Bartels, unpublished). Another way of stabilizing the virus is to grind each 100 g infected tissue in 100 ml phosphate buffer (pH 7.0) containing 10 g Al2O3 (Fribourg & de Zoeten, 1970).
Properties of Particles
No reports.
Particle Structure
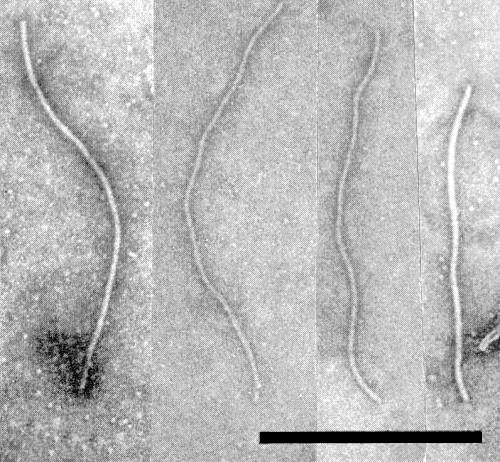
Particles are flexuous filaments (Fig. 5) with normal length c. 730 nm, diameter c. 15 nm (Brandes & Paul, 1957).
Particle Composition
No reports.
Relations with Cells and Tissues
No intracellular inclusions found in infected potato plants (Bawden & Sheffield, 1944).
Notes
Potato virus A and potato virus Y both infect potato, have particles indistinguishable by electron microscopy, and are difficult to distinguish by the symptoms they produce in many test plants. However, they may be distinguished serologically and only potato virus A produces local lesions in Solanum demissum SdA.
Figures
References list for DPV: Potato virus A (54)
- Bartels, Phytopath. Z. 21: 395, 1954.
- Bartels, Phytopath. Z. 49: 257, 1964.
- Bartels, Potato Res. 13: 119, 1970.
- Bawden & Sheffield, Ann. appl. Biol. 31: 33, 1944.
- Brandes, Mitt. biol. BundAnst. Ld- u. Forstw. 110, 130 pp., 1964.
- Brandes & Paul, Arch. Mikrobiol. 26: 358, 1957.
- Calvert, Pl. Path. 9: 144, 1960.
- Clinch, Loughnane & Murphy, Scient. Proc. R. Dubl. Soc. 21: 431, 1936.
- Cockerham, Proc. 3rd Conf. Potato Virus Diseases, Lisse-Wageningen, 1957: 199, 1958.
- Fribourg & de Zoeten, Phytopathology 60: 1415, 1970.
- Hollings & Stone, Ann. appl. Biol. 65: 411, 1970.
- Köhler, Züchter 23: 173, 1953.
- MacLachlan, Larson & Walker, Res. Bull. agric. Exp. Stn Univ. Wis. 180, 36pp., 1953.
- Murphy & McKay, Scient. Proc. R. Dubl. Soc. 20: 347, 1932.
- Schmelzer, Naturwissenschaften 46: 83, 1959.
- Sylvester, Hilgardia 23: 53, 1954.