Details of DPV and References
DPV NO: 55 June 1971
Family: Potyviridae
Genus: Potyvirus
Species: Tobacco etch virus | Acronym: TEV
There is a more recent description of this virus: DPV 258
Tobacco etch virus
R. J. Shepherd Department of Plant Pathology, University of California, Davis, California, USA
D. E. Purcifull Plant Pathology Department, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, Florida, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Johnson (1930).
Selected synonyms
- Marmor erodens
(Rev. appl. Mycol. 28: 514) - Tobacco virus 13 (Rev. appl. Mycol. 17: 52)
- Tomato etch virus (Rev. appl. Mycol. 20: 85)
- Nicotiana virus 7 (Rev. appl. Mycol. 17: 52)
- Tomato etch virus (Rev. appl. Mycol. 20: 85)
An RNA-containing virus of the potato virus Y group with flexuous filamentous particles c. 730 nm in length. It is found mainly in solanaceous plants, is transmitted in the non-persistent manner by aphids and is readily transmissible by inoculation of sap.
Main Diseases
Causes necrotic and/or chlorotic mottle diseases of tobacco, pepper, tomato and other solanaceous plants.
Geographical Distribution
Common in North and South America.
Host Range and Symptomatology
Species in about 15 dicotyledonous families are susceptible to infection (Holmes, 1946; Schmelzer, 1967). Usually confined to solanaceous plants in nature. Mechanically transmissible to the following:
-
Diagnostic species
- Nicotiana tabacum
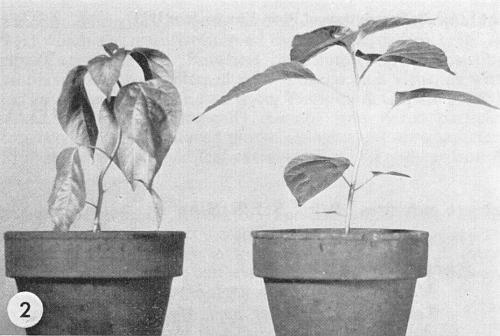
(tobacco). Inoculated leaves usually develop indefinite chlorotic spots or necrotic rings or arcs. Systemic symptoms are vein-clearing and necrotic etching (Fig. 1) followed by chlorotic mottle; virulent strains cause necrotic spots and distortion. Some strains produce mild interveinal mottle with green vein-banding. - Capsicum annuum (pepper). Systemic chlorotic mottle with distortion in
many varieties. Most
strains cause root necrosis, wilt and death of C. frutescens cv.
Tabasco
(Fig. 2)
(Greenleaf, 1953).
- Datura stramonium. Mottle, vein-banding, and leaf distortion (Fig. 3) in systemically infected leaves.
- Lycopersicon esculentum (tomato). Mild distortion, inconspicuous mottle.
- Datura stramonium. Mottle, vein-banding, and leaf distortion (Fig. 3) in systemically infected leaves.
-
Propagation species
- N. glutinosa
or varieties of N. tabacum hypersensitive to tobacco mosaic virus are suitable for maintaining cultures and are good sources of virus for purification.Assay species
- The virus causes local lesions in Chenopodium album, C. amaranticolor (Fig. 4), C. quinoa and Physalis peruviana. N. tabacum is preferable as an assay host when using aphid vectors.
Strains
Variants differing in virulence towards tobacco and other hosts are common. Variants called ‘severe etch’ induce more prominent stunting, chlorosis, and necrosis than ‘mild etch’ variants (Johnson, 1930; Bawden & Kassanis, 1941).
Transmission by Vectors
Transmissible in the non-persistent manner by more than 10 species of aphids (Kennedy, Day & Eastop, 1962; Laird & Dickson, 1963), notably Myzus persicae. All instars can transmit. Acquisition and inoculation can occur in 1-2 min. No latent period. Virus is retained for only 1-4 h by feeding aphids. No transmission to progeny aphids (Kassanis, 1941).
Transmission through Seed
None reported.
Transmission by Dodder
Transmitted by Cuscuta californica but not by two other species of Cuscuta (Bennett, 1944).
Serology
The virus is strongly immunogenic. Tube or micro-precipitin tests with clarified sap or partially purified virus preparations are the most useful because the virus does not diffuse well in agar gels. Preparations containing intact virus particles give flagellar type precipitates in tests in liquid. Immunodiffusion tests have been used for diagnosis and for tests for relationship with other viruses, using alkali-treated (pH 10.5) virus as antigen (Purcifull & Shepherd, 1964; Purcifull & Gooding, 1970). The virus has also been diagnosed serologically in crude extracts using agar gels containing sodium dodecyl sulphate (Gooding & Bing, 1970).
Relationships
Particle length and other biological and physical properties place tobacco etch virus in the potato virus Y group of viruses and it is probably distantly serologically related to other viruses in this group, e.g. potato Y, henbane mosaic and Colombian datura viruses (Bartels, 1964; Kahn & Bartels, 1968; Purcifull & Gooding, 1970). Pokeweed mosaic virus may be serologically related (Shepherd, Fulton & Wakeman, 1969). In plant-protection tests, strains protect against the effects of one another and may or may not protect against the effects of those viruses that are distantly serologically related (Bawden & Kassanis, 1945; Schmelzer, Bartels & Klinkowski, 1960).
Stability in Sap
In tobacco sap, the thermal inactivation point (10 min) is about 55°C, dilution end-point about l0-4. Infectivity of sap survives for 5-10 days at 20°C.
Purification
Of the various methods described, the following two are probably best for
avoiding aggregation
and insolubility of virus. Systemically infected tobacco may yield 30-60 mg virus
per litre of sap.
1. Purcifull (1966).
Homogenize tissue in 0.5 M phosphate buffer (pH 7.5) containing 1% sodium
sulphite. Filter and incubate extract at 4°C for 8-16 h. Clarify extract by
adding n-butanol
(1 ml per 10 ml extract) and centrifuge at low speed. Sediment virus from the
clarified extract by
centrifuging at high speed, and resuspending the pellets in 0.02 M borate buffer,
pH 8.2. Precipitate
virus by adding NaCl to 0.5% (w/v) and adjusting to pH 4.8. Remove virus by
low speed centrifugation
and resuspend in 0.02 M borate; further purify by two cycles of differential centrifugation.
2. Damirdagh & Shepherd (1970a). Homogenize in 0.5 M phosphate buffer (pH 7.1, containing 1% 2-mercaptoethanol) using 120 ml buffer per 100 g tissue. Clarify by adding 1/10th volume n-butanol and centrifuge at low speed. Precipitate virus from clarified extract by adding 4 g polyethylene glycol (6000-7000 M. Wt) per 100 ml extract and centrifuge at low speed. Resuspend virus in 0.025 M phosphate (pH 7.4) containing 0.5-1.0 M urea and 0.1% 2-mercaptoethanol and subject to 2 cycles of differential centrifugation, resuspending the virus in this solution at each step. To each 3.2 ml of virus solution add 1.8 ml of saturated CsCl (at 4°C) in 0.025 M phosphate, pH 7.4, and centrifuge for 24 h at high speed in an angle or swinging-bucket rotor. Remove the virus, which bands in about the centre of these equilibrium density gradients, and dialyse against 0.005 M glycylglycine buffer, pH 7.8.
Properties of Particles
Sedimentation coefficient (s20, w) at infinite dilution: 154 S. No accessory components are found by analytical ultracentrifugation.
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 2.4 when virus is mostly unaggregated.
A260/A280: about 1.27-1.30.
Buoyant density: 1.33 (in CsCl).
Particle Structure
Particles are flexuous filaments (Fig. 5) of diameter 12-13 nm and length 730 nm (Brandes & Wetter, 1959).
Particle Composition
RNA: Single-stranded. Molar percentages of nucleotides: G23; A30; C20; U27. RNA is 5% of particle weight (Damirdagh & Shepherd, 1970b).
Protein: 95% of particle weight (Damirdagh & Shepherd, 1970b).
Relations with Cells and Tissues
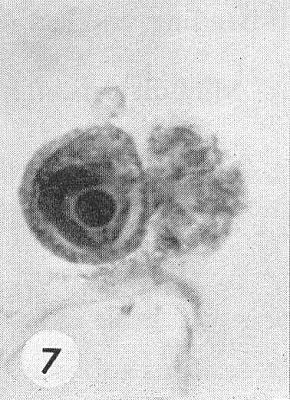
The virus induces nuclear crystals and cytoplasmic inclusions (Kassanis, 1939; Sheffield, 1941) which are readily observed by light microscopy (Fig. 7), especially when stained in epidermal strips (Christie, 1967). ‘Pinwheel’ inclusions (Fig. 6) consisting of striated lamellae are found in the cytoplasm of tissues infected with tobacco etch virus, as with other viruses of the potato virus Y group (Edwardson, 1966; Edwardson, Purcifull & Christie, 1968). These proteinaceous inclusions are structurally and serologically distinct from virus particles (Hiebert et al., 1971). The lamellae of tobacco etch virus-induced pinwheel inclusions are characteristically seen as striated triangles (Fig. 8) in negatively stained leaf extracts (Purcifull, Edwardson & Christie, 1970).
Notes
Several members of the potato Y group of viruses, e.g. potato Y and henbane mosaic viruses, occur in solanaceous plants and are easily confused with tobacco etch virus. Characteristic reactions induced in tobacco and pepper varieties, susceptibility of Datura stramonium, production of intra-nuclear inclusions, presence of triangular striated lamellae in leaf extracts, and serological tests are helpful in diagnosis of the virus.
Figures
References list for DPV: Tobacco etch virus (55)
- Bartels, Phytopath. Z. 49: 257, 1964.
- Bawden & Kassanis, Ann. appl. Biol. 28: 107, 1941.
- Bawden & Kassanis, Ann. appl. Biol. 32: 52, 1945.
- Bennett, Phytopathology 34: 905, 1944.
- Brandes & Wetter, Virology 8: 99, 1959.
- Christie, Virology 31: 268, 1967.
- Damirdagh & Shepherd, Phytopathology 60: 132, 1970a.
- Damirdagh & Shepherd, Virology 40: 84, 1970b.
- Edwardson, Am. J. Bot. 53: 359, 1966.
- Edwardson, Purcifull & Christie, Virology 34: 250, 1968.
- Gooding & Bing, Phytopathology 60: 1293, 1970.
- Greenleaf, Phytopathology 43: 564, 1953.
- Hiebert, Purcifull, R. Christie & S. Christie, Virology 43: 688, 1971.
- Holmes, Phytopathology 36: 643, 1946.
- Johnson, Bull. Ky agric. Exp. Stn 306: 289, 1930.
- Kahn & Bartels, Phytopathology 58: 587, 1968.
- Kassanis, Ann. appl. Biol. 26: 705, 1939.
- Kassanis, Ann. appl. Biol. 28: 238, 1941.
- Kennedy, Day & Eastop, A conspectus of aphids as vectors of plant viruses, London, Commonwealth Institute of Entomology, 1962.
- Laird & Dickson, Phytopathology 53: 48, 1963.
- Purcifull, Virology 29: 8, 1966.
- Purcifull & Gooding, Phytopathology 60: 1036, 1970.
- Purcifull & Shepherd, Phytopathology 54: 1102, 1964.
- Purcifull, Edwardson & Christie, Phytopathology 60: 779, 1970.
- Schmelzer, Phytopath. Z. 60: 301, 1967.
- Schmelzer, Bartels & Klinkowski, Phytopath. Z. 40: 52, 1960.
- Sheffield, Jl R. microsc. Soc. 61: 30, 1941.
- Shepherd, Fulton & Wakeman, Phytopathology 59: 219, 1969.