Details of DPV and References
DPV NO: 71 October 1971
Family: Potyviridae
Genus: Potyvirus
Species: Tulip breaking virus | Acronym: TBV
Tulip breaking virus
D. H. M. van Slogteren Bulb Research Centre, Lisse, The Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Cayley (1928) and McKay, Brierley & Dykstra (1929).
- Selected synonyms
- Tulip mosaic virus (Rev. appl. Mycol. 36: 303)
- Tulipa virus 1 (Rev. appl. Mycol. 36: 303)
- Marmor tulipae (Rev. appl. Mycol. 28: 514)
- Tulipavirus vulgare (Rev. appl. Mycol. 38: 677)
- Lily mottle virus (Rev. appl. Mycol. 24: 59)
- Tulipa virus 1 (Rev. appl. Mycol. 36: 303)
- A virus with flexuous filamentous particles, c. 740 nm long, sap-transmissible to two genera of Liliaceae and transmitted by a few species of aphids in the non-persistent manner. Causes colour ‘breaking’ in the perianth of tulips. World-wide distribution.
Main Diseases
Causes flower ‘breaking’ in red and purple varieties of tulip species and their hybrids; it affects the amount of anthocyanin in the vacuoles of epidermal cells in the petals (Dufrénoy, 1931), resulting in different types of colour-breaks depending on the variety of tulip and on the strain of the virus. White- and yellow-flowered varieties are incapable of breaking because they lack anthocyanins, their colour being determined by colourless or yellow plastids in the mesophyll. Infected plants may also show leaf mottle (Fig. 5). Colour-breaking has been known since tulips were introduced to Europe in the 16th century. Paintings of the 16th and 17th century often include tulips and these are always of the broken type. Although in the 17th century broken tulips were highly valued, nowadays they are not, because infected plants are no longer true to varietal type and the production of offsets is decreased. The virus is also found in lily species, causing mild to moderate mottling in the leaves.
Geographical Distribution
World-wide. Reported in all temperate regions where tulips are grown; particularly common in southern Europe where the aphid vectors are abundant early in the growing season.
Host Range and Symptomatology
Reported to infect only two plant genera, Tulipa and Lilium, both in the Liliaceae. Transmissible by inoculation of sap and by bulb-grafting. As early as 1637 Dutch growers produced new varieties of tulip with flower breaks by grafting tulip bulbs with broken flowers to bulbs with uniformly coloured flowers (Hoog, 1933).
- Diagnostic species
- Lilium formosanum.
Mottle or streak symptoms in the tip leaves about 2 weeks after mechanically inoculating seedlings with extracts from petals, leaves or bulb scales of infected tulip. Leaves and flowers produced later are distorted (Brierly & Smith, 1944; Yamaguchi, 1958).- Propagation species
- The tulip varieties Croisette, Grand Pride and Paljas are good sources for virus
purification.
- Assay species
- No local lesion host reported.
Strains
Severe strains (STBV) and mild strains (MTBV) have been distinguished by the type and severity of flower break symptoms they cause (van Slogteren & de Vos, 1966; van Slogteren & Asjes, 1970). In certain varieties STBV causes ‘full break’ symptoms, in which anthocyanins fail to form in parts of the petals so that the colour of the mesophyll (either white or yellow) is exposed (Fig. 1). In the same varieties MTBV causes ‘self break’ symptoms in which the colour in parts of the petals is intensified because anthocyanins are formed in excess (Fig. 2). The most common type of breaking found in naturally infected plants, called ‘average break’, is caused by infection with a mixture of STBV and MTBV; both ‘full break’ and ‘self break’ symptoms are present, together with some unbroken areas, in different parts of the same petal (Fig. 3). Plants infected with a mixture of strains when young (sprouts about 3 cm long) may develop atypical symptoms in the current season (Fig. 4), with ‘full break’ restricted to the basal part of the petal and severe ‘self break’ in the upper parts (van Slogteren & de Bruyn Ouboter, 1941; Yamaguchi, 1961). Other varieties were found by McWhorter (1938) to be ‘incapable of full breaking’; these always show ‘self break’ symptoms whether infected with STBV or MTBV or a mixture of both strains.
Transmission by Vectors
Transmitted by at least 4 species of aphids in the non-persistent manner. Of these Myzus persicae is the most efficient; the others are Aphis gossypii, Doralis fabae and Macrosiphum euphorbiae. McKenny-Hughes (1931, 1934) reported that Yezabura tulipae transmits between stored tulip bulbs, but this has not been confirmed by others (Brierley & McKay, 1938; van Slogteren & de Bruyn Ouboter, 1941). Transmission by Myzus persicae is enhanced when the aphid is starved for 1-2 hr before the acquisition access feed, the optimum acquisition period being 2-5 min.
Transmission through Seed
Not reported.
Transmission by Dodder
Not reported.
Serology
A good immunogen. Antisera with titres greater than 1/1000 have been prepared against different isolates (van Slogteren & de Vos, 1966). For microprecipitin tests the virus is concentrated by ammonium sulphate precipitation. Dilution end-points are increased by immersing infected leaves in water at 50°C before extracting the sap. Precipitates are of the flocculent (flagellar) type.
Relationships
STBV reacted with both the homologous antiserum and with antiserum to MTBV, but MTBV reacted with neither antiserum, presumably because too little virus was present (van Slogteren & de Vos, 1966). An observation by McWhorter (1938) suggests that MTBV does not protect against super-infection with STBV: he inoculated tulips showing ‘self break’ with STBV and 5 out of 9 plants then developed ‘full break’.
Tulip breaking virus is a member of the potato virus Y group (Brandes & Wetter, 1959); Bartels (1971) found a distant serological relationship to tobacco etch virus, cross-reactions with both heterologous antisera being positive. Also, tulip breaking virus reacted with antiserum to henbane mosaic virus but henbane mosaic virus did not react with antiserum to tulip breaking virus.
Stability in Sap
Infectivity in tulip sap is lost after 10 min at 65-70°C and after dilution beyond 10-5 (McWhorter, 1935). In lily sap it is retained for 4-6 days at 18°C (Brierley & Smith, 1944).
Purification
Attempts to obtain highly purified virus preparations have failed because of the small amounts of virus in leaf extracts and because the particles aggregate. Preparations for immunizing rabbits were partially purified by a method used for potato virus M (Rozendaal & van Slogteren, 1958).
Properties of Particles
No reports.
Particle Structure
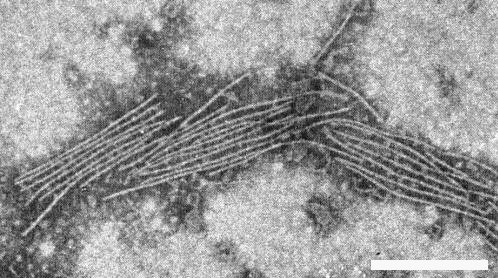
Particles are flexuous filaments (Fig. 6) c. 14 nm wide and with a modal particle length of 740 nm (Brandes & Bercks, 1965).
Particle Composition
No reports.
Relations with Cells and Tissues
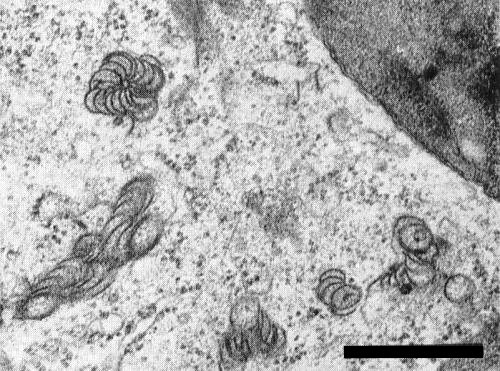
McWhorter (1941), using light microscopy, found two kinds of intracellular inclusions in epidermal cells of infected tulip leaves. Using electron microscopy of ultra-thin sections, Yamaguchi, Kikumoto & Matsui (1963) observed bundles of parallel particles in infected tulip petals, and T. C. Allen (pers. comm.) found ‘pinwheel’ inclusions in infected cells of both tulip and lily (Fig. 7).
Notes
Tulips infected with cucumber mosaic virus show a flower break which might be confused with that caused by tulip breaking virus but is usually distinctive because it is confined to the margins of the petals (van Slogteren, 1966). In lilies, tulip breaking virus is sometimes found together with lily symptomless virus (van Slogteren, unpublished), a virus described earlier by Civerolo, Semancik & Weathers (1968) and by Allen & Lyons (1969).
Brierley & Smith (1944) reported four additional hosts of tulip breaking virus in the Liliaceae (Calochortus sp., Fritillaria pudica, Zygadenus fremontii and Ornithogalum thyrsoides). However, this report needs confirming because the virus used in these experiments came from lily and may have been contaminated with lily symptomless virus.
Acknowledgements
Figs 1-5 courtesy of Bulb Research Centre, Lisse; Fig. 6 courtesy of C. J. Asjes, Bulb Research Centre, Lisse; Fig. 7 courtesy of T. C. Allen, Oregon State University, Corvallis, Oregon, USA.
Figures
References list for DPV: Tulip breaking virus (71)
- Allen & Lyons, Phytopathology 59: 1318, 1969.
- Bartels, Phytopath. Z. 71: 87, 1971.
- Brandes & Bercks, Adv. Virus Res. 11: 1, 1965.
- Brandes & Wetter, Virology 8: 99, 1959.
- Brierley & McKay, Phytopathology 28: 123, 1938.
- Brierley & Smith, Phytopathology 34: 718, 1944.
- Cayley, Gdnrs' Chron. 83: 435, 1928.
- Civerolo, Semancik & Weathers, Phytopathology 58: 1481, 1968.
- Dufrénoy, C. r. Séanc. Soc. Biol. 108: 51, 1931.
- Hoog, Gdnrs' Chron. 94: 471, 1933.
- McKay, Brierley & Dykstra, Yb. U.S. Dep. Agric. 1928: 596, 1929.
- McKenny-Hughes, Ann. appl. Biol. 18: 16, 1931.
- McKenny-Hughes, Ann. appl. Biol. 21: 112, 1934.
- McWhorter, Phytopathology 25: 898, 1935.
- McWhorter, Ann. appl. Biol. 25: 254, 1938.
- McWhorter, Stain Technol. 16: 143, 1941.
- Rozendaal & van Slogteren, Proc. 3rd Conf. Potato Virus Diseases, Lisse-Wageningen, 1957: 20, 1958.
- van Slogteren, Meded. Rijksfac. Landb. Wetensch. Gent 31: 986, 1966.
- van Slogteren & Asjes, Daffodil Tulip Yb. 35: 85, 1970.
- van Slogteren & de Bruyn Ouboter, Meded. LandbHoogesch. Wageningen 45(4), 54 pp., 1941.
- van Slogteren & de Vos, Proc. Conf. Pl. Viruses, Wageningen 1965: 320, 1966.
- Wetter, Arch. Mikrobiol. 37: 278, 1960.
- Yamaguchi, Ann. phytopath. Soc. Japan 23: 240, 1958.
- Yamaguchi, Ann. phytopath. Soc. Japan 26: 131, 1961.
- Yamaguchi, Kikumoto & Matsui, Virology 20: 143, 1963.