Details of DPV and References
DPV NO: 84 June 1972
Family: Potyviridae
Genus: Potyvirus
Species: Papaya ringspot virus | Acronym: PRSV
There is a more recent description of this virus: DPV 292
Papaya ringspot virus
D. E. Purcifull Plant Virus Laboratory, Plant Pathology Department, University of Florida, Gainesville, Florida, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Jensen (1949a),
Conover (1962,
1964a),
De Bokx (1965) and
Zettler, Edwardson & Purcifull (1968).
Synonyms
- The following incompletely studied viruses may be synonyms:
- Papaya (papaw) distortion ringspot virus (Rev. appl. Mycol. 41: 530)
- Papaya (papaw) mosaic virus (Rev. appl. Mycol. 28: 131)
-
A virus with flexuous, filamentous particles about 800 nm long. It is stylet-borne by aphids, is sap-transmissible, and has a narrow host range. Causes a disease of major importance in papaya.
Main Diseases
In papaya, it causes mottling and distortion of leaves, rings and spots on fruit, and streaks on stems and petioles. It stunts the plants and diminishes fruit production (Conover, 1964a).
Geographical Distribution
In most tropical and subtropical areas of the world where papayas are grown.
Host Range and Symptomatology
Host range is narrow; 11 species in three dicotyledonous families (Caricaceae, Chenopodiaceae and Cucurbitaceae) have been experimentally infected, but papaya is the only reported natural host. Sap-transmissible.
-
Diagnostic species
- Carica papaya
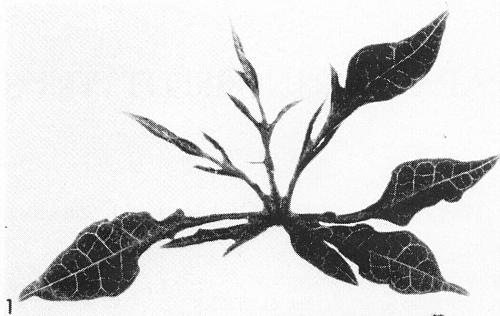
(papaya). Symptoms in papaya are variable. Seedlings show vein-clearing and downward cupping of the young systemically infected leaves about 2 weeks after inoculation. After several weeks, leaves may become mottled (Fig. 3) and deformed, the lobes being markedly reduced in size (Fig. 1). Among other species of Carica tested, some were susceptible and some were not (Conover, 1964a). - Cucurbita pepo (summer squash and pumpkin). Yellow chlorotic areas appear along minor veins in systemically infected squash leaves 10-14 days after inoculation (Fig. 5), followed by mottle and some leaf distortion. Symptoms in pumpkin are similar, but generally milder.
-
Propagation species
- Papaya, squash and pumpkin are useful for maintaining cultures. Pumpkin has been used as a source of virus for purification (Milne & Grogan, 1969).
-
Assay species
- Chenopodium amaranticolor
develops local lesions (Cook & Milbrath, 1971). Pumpkin is useful for vector studies.
Strains
A minor variant designated ‘faint mottle ringspot’ induces milder symptoms in Carica papaya than does the strain common in Florida (Conover, 1964a, 1964b).
Transmission by Vectors
Transmitted by several species of aphids in a non-persistent manner, notably Myzus persicae (Jensen, 1949b; Conover, 1964a; Zettler et al., 1968). Other vectors are Aphis gossypii, A. medicaginis, A. rumicis, Macrosiphum solanifolii and Micromyzus formosanus (Jensen, 1949b).
Transmission through Seed
None detected in papaya (De Bokx, 1965).
Transmission by Dodder
No information.
Serology
An antiserum was obtained by injecting rabbits with clarified sap from infected papaya leaves (Story & Halliwell, 1969b). Liquid precipitin tests with purified virus and immunodiffusion tests with detergent-treated virus are useful for studying serological relationships (Milne & Grogan, 1969).
Relationships
The virus is serologically related to watermelon mosaic virus (Milne & Grogan, 1969) but the degree of relationship is not clear; its general properties place it in the potato virus Y group of viruses (Brandes & Bercks, 1965).
Stability in Sap
In papaya sap, the virus loses infectivity after 10 min at 54-56°C and after 8 hr at room temperature. The dilution end-point is about l0-3 (Conover, 1964a).
Purification
Partially purified virus for serological tests was obtained from infected pumpkin leaves using a method developed for watermelon mosaic virus (Milne & Grogan, 1969). Tissue was homogenized in 0.3 M potassium phosphate buffer, pH 7.6 (2 ml/g tissue). The extract was clarified with n-butanol and the virus obtained by two cycles of differential centrifugation.
Properties of Particles
No information.
Particle Structure
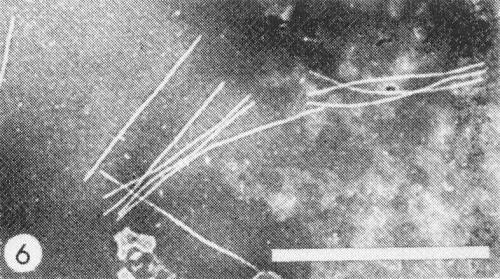
Flexuous filaments (Fig. 6) 800 nm long and 12 nm in diameter (Herold & Weibel, 1962).
Particle Composition
No information.
Relations with Cells and Tissues
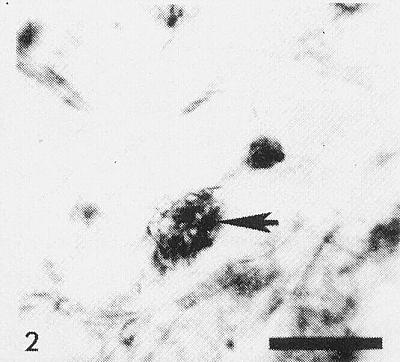
Cytoplasmic inclusions (Fig. 2) have been observed in stained epidermal strips of squash (Christie, 1971). Pinwheel inclusions (Fig. 4) are seen in the cytoplasm of infected tissues (Zettler et al., 1968; Story & Halliwell, 1969b). In negatively stained extracts from infected leaves the striated nature of these inclusions is revealed (Fig. 7). Virus particles have been observed in the cytoplasm of infected cells (Herold & Weibel, 1962).
Notes
Numerous mosaic virus diseases of papaya have been reported from various parts of the world (Adsuar, 1947; Capoor & Varma, 1958; Ishii & Holtzmann, 1963; Kulkarni, 1970; Namba & Kawanishi, 1966; Pozdena, 1967; Singh, 1969). They resemble papaya ringspot in some respects, but the precise relationship of the causal viruses has not been determined. They should not be confused, however, with the papaya mosaic virus described by Conover (1962, 1964b), De Bokx (1965) and Zettler et al. (1968). Papaya mosaic virus is a flexuous rod 530 nm long, is not aphid-transmitted, and is a member of the potato virus X group (Brandes & Bercks, 1965). Watermelon mosaic virus, to which papaya ringspot virus is serologically related, does not infect papaya (Milne & Grogan, 1969).
A mycoplasma-like organism has been associated with the leafhopper-borne bunchy top disease of papaya (Story & Halliwell, 1969a). The virus diseases of papaya have been reviewed recently by Cook (1972).
Figures
References list for DPV: Papaya ringspot virus (84)
- Adsuar, J. Agric. Univ. P. Rico 31: 248, 1947.
- Brandes & Bercks, Adv. Virus Res. 11: 1, 1965.
- Capoor & Varma, Indian J. agric. Sci. 28: 225, 1958.
- Christie, Proc. Fla Pest Control Conf. 5: 65, 1971.
- Conover, Phytopathology 52: 6, 1962.
- Conover, Proc. Fla St. hort. Soc. 77: 440, 1964a.
- Conover, Proc. Fla St. hort. Soc. 77: 444, 1964b.
- Cook, Tech. Bull. Fla agric. Exp. Stn 750, 19 pp., 1972.
- Cook & Milbrath, Pl. Dis. Reptr 55: 785, 1971.
- De Bokx, Pl. Dis. Reptr 49: 742, 1965.
- Herold & Weibel, Virology 18: 302, 1962.
- Ishii & Holtzmann, Pl. Dis. Reptr 47: 947, 1963.
- Jensen, Phytopathology 39: 191, 1949a.
- Jensen, Phytopathology 39: 212, 1949b.
- Kulkarni, Ann. appl. Biol. 66: 1, 1970.
- Milne & Grogan, Phytopathology 59: 809, 1969.
- Namba & Kawanishi, J. econ. Ent. 59: 669, 1966.
- Pozdena, Proc. Sixth Conf. Czechoslovak Plant Virologists, Prague, 1967.
- Singh, Pl. Dis. Reptr 53: 267, 1969.
- Story & Halliwell, Phytopathology 59: 1336, 1969a.
- Story & Halliwell, Pl. Dis. Reptr 53: 757, 1969b.
- Zettler, Edwardson & Purcifull, Phytopathology 58: 332, 1968.