Details of DPV and References
DPV NO: 89 June 1972
Family: Luteoviridae
Genus: Polerovirus
Species: Beet western yellows virus | Acronym: BWYV
Beet western yellows virus
J. E. Duffus US Agricultural Research Station, Salinas, California 93901, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Duffus (1960, 1961).
- Selected synonyms
- Turnip yellows virus (Rev. appl. Mycol. 32: 294)
- Malva yellows virus (Rev. appl. Mycol. 39: 761)
- Radish yellows virus (Rev. appl. Mycol. 39: 753)
- Turnip mild yellows virus (Rev. appl. Mycol. 42: 651)
- Malva yellows virus (Rev. appl. Mycol. 39: 761)
A virus with isometric particles c. 26 nm in diameter. Transmitted by several species of aphids in the persistent (circulative) manner, but not by sap inoculation. It has a wide host range among commercially important crop plants. Distribution probably world-wide.
Main Diseases
Causes stunting and chlorosis of a wide range of dicotyledonous species, including sugar beet, red beet, spinach, lettuce, broccoli, cauliflower, radish, turnip and flax.
Geographical Distribution
Reported from North America, Europe (Duffus & Russell, 1970) and Asia (Sugimoto, Murayama & Sanai, 1970); probably common throughout the world.
Host Range and Symptomatology
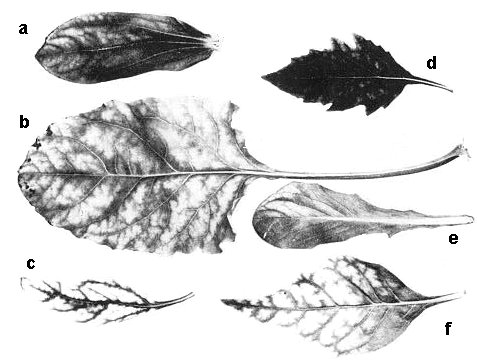
Host range is wide; over 100 species in 21 dicotyledonous families are susceptible to various strains of the virus (Duffus, 1960). Occurs naturally in a number of common weed species and often in common overwintering hosts of vector aphids (Wallis, 1967). Transmitted readily by aphids but not by sap inoculation. Interveinal yellowing of the older or intermediate leaves, especially under high light intensity, is the most common symptom (Fig. 1).
- Diagnostic species
- Capsella bursa-pastoris. Severe chlorosis and
moderate leaf curl, thick and brittle leaves (Fig. 2);
yellowing develops acropetally. On some biotypes of
Capsella, purpling or reddening accompanies the chlorosis.
- Senecio vulgaris. Purple coloration of the margins of mature leaves.
- Claytonia perfoliata. Pink to salmon coloration of the edges of older leaves.
- Senecio vulgaris. Purple coloration of the margins of mature leaves.
- Propagation species
- Beta vulgaris (sugar beet), Raphanus sativus (radish) and Capsella bursa-pastoris are suitable for maintaining cultures; and the two last species as sources of virus for purification.
- Assay species
- Capsella bursa-pastoris, by determining the proportion of seedlings that become infected. The plants should be inoculated by means of single aphids given 24 hr acquisition feeds on infected plants or plant extracts.
Strains
Many variants have been distinguished on the basis of host range and virulence (Duffus, 1964). Different variants seem to predominate in different plant species, e.g. malva yellows virus (Costa, Duffus & Bardin, 1959) in Malva, turnip yellows virus (Vanderwalle & Roland, 1951) in turnip. Different variants may induce similar reactions in some plant species but distinct reactions in others in regard to susceptibility, stunting and yellowing. All isolates so far tested infect Capsella bursa-pastoris, Claytonia perfoliata and Senecio vulgaris. The variants do not cross-protect (Duffus & Gold, 1969).
Transmission by Vectors
Transmissible by 8 species of aphids, the most important of which is Myzus persicae. Transmission is in the persistent (circulative) manner; the virus persists in the vector for over 50 days. Vectors retain ability to transmit after moulting, but the virus is not transmitted to their progeny. Minimum acquisition feeding period, 5 min; minimum inoculation feeding period, 10 min. There is a latent period of 12-24 hr. Some differences between virus isolates in their efficiency of transmission by vectors are related to differences in virus concentration in the plant hosts (J. E. Duffus, unpublished).
Transmission through Seed
Not known to occur.
Transmission by Dodder
Not known to occur.
Serology
The virus is strongly immunogenic but occurs in low concentrations in plants and partially purified preparations must be used in serological tests. Serological neutralization of infectivity, demonstrated by feeding insects through membranes on virus-antiserum mixtures, has been successful with virus or antiserum concentrations too low for conventional serological techniques (Duffus & Gold, 1965, 1969; Gold & Duffus, 1967).
Relationships
A number of viruses transmitted in the persistent manner by Myzus persicae have certain host plants and host reactions in common (beet western yellows, beet mild yellowing, malva yellows, potato leaf roll, turnip latent and turnip yellows viruses). Using antiserum to beet western yellows virus in infectivity neutralization tests, malva yellows and turnip yellows viruses have been shown to be closely related to beet western yellows virus; however, potato leaf roll virus appears not to be serologically related (Duffus & Gold, 1969; J. E. Duffus & G. E. Russell, unpublished). Beet mild yellowing virus (Russell, 1958), produces similar symptoms in certain key indicator hosts and is transmitted in a similar way by vectors but differs greatly in epidemiology and host range from typical isolates of beet western yellows virus (Russell, 1963, 1965; Björling & Nilsson, 1966). No tests of the serological relationship between beet mild yellowing and beet western yellows viruses have been made and it would seem best at present to regard these viruses as separate.
Stability in Sap
Determined for several isolates by feeding aphids through membranes on partially purified virus preparations. Thermal inactivation point (10 min) is about 65°C; dilution end-point of unconcentrated sap 1/8; extracts were infectious after 16 days at 24°C. Alternate freezing and thawing at 4 or 5 day intervals for 1 month had no effect on virus activity. Infectivity withstood drying for at least 3 years (Duffus, 1969).
Purification
Several strains have been purified by chloroform clarification, differential centrifugation, density gradient centrifugation and density gradient electrophoresis (Smith, Duffus & Gold, 1966; Gold & Duffus, 1967).
Properties of Particles
Not known.
Particle Structure
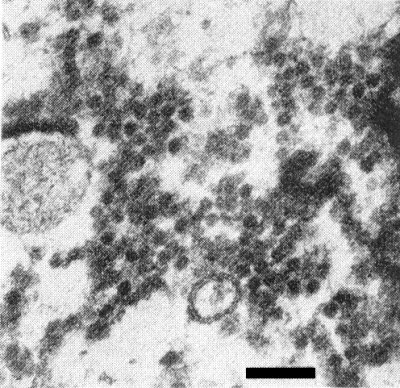
Particles are isometric; diameter about 26 nm in thin sections of host tissue (Fig. 3) Esau & Hoefert, 1972a; Ruppel, 1968).
Particle Composition
Not known.
Relations with Cells and Tissues
Particles appear to be confined to the phloem and associated with phloem degeneration. They may occur in nuclei of phloem parenchyma cells (Esau & Hoefert, 1972b).
Notes
Leaves of infected beet plants are more susceptible to attack by Alternaria species than are healthy plants or plants infected with beet yellows virus (J. E. Duffus, unpublished).
Figures
References list for DPV: Beet western yellows virus (89)
- Björling & Nilsson, Socker 21: 1, 1966.
- Costa, Duffus & Bardin, J. Am. Soc. Sug. Beet Technol. 10: 371, 1959.
- Duffus, Phytopathology 50: 389, 1960.
- Duffus, Phytopathology 51: 605, 1961.
- Duffus, Phytopathology 54: 736, 1964.
- Duffus, Phytopathology 59: 1668, 1969.
- Duffus & Gold, Virology 27: 388, 1965.
- Duffus & Gold, Virology 37: 150, 1969.
- Duffus & Russell, Phytopathology 60: 1199, 1970.
- Esau & Hoefert, J. Ultrastruct. Res. 40: 556, 1972a.
- Esau & Hoefert, Virology 48: 724, 1972b.
- Gold & Duffus, Virology 31: 308, 1967.
- Ruppel, Phytopathology 58: 256, 1968.
- Russell, Ann. appl. Biol. 46: 393, 1958.
- Russell, Ann. appl. Biol. 52: 405, 1963.
- Russell, Ann. appl. Biol. 55: 245, 1965.
- Smith, Duffus & Gold, Phytopathology 56: 902, 1966.
- Sugimoto, Murayama & Sanai, Bull. Sugar Beet Res. 8: 1, 1970.
- Vanderwalle & Roland, Parasitica 7: 14, 1951.
- Wallis, J. econ. Ent. 60: 904, 1967.