Details of DPV and References
DPV NO: 50 June 1971
Family: Potyviridae
Genus: Potyvirus
Species: Celery mosaic virus | Acronym: CeMV
Celery mosaic virus
J. F. Shepard Department of Botany & Microbiology, Montana State University, Bozeman, Montana 59715, USA
R. G. Grogan Department of Plant Pathology, University of California, Davis, California 95616, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described and named western celery mosaic virus by Severin & Freitag, 1938.
- Selected synonyms
- Apium
virus 1 (Rev. appl. Mycol. 17: 52) - Marmor umbelliferarum (Rev. appl. Mycol. 28: 514)
- Western celery mosaic virus (Rev. appl. Mycol. 18: 369)
- Western celery mosaic virus (Rev. appl. Mycol. 18: 369)
- A virus with flexuous filamentous particles about 780 nm in length; sap-transmissible to a narrow range of hosts, and transmitted by several species of aphids.
Main Diseases
Causes a mosaic disease of celery. In Britain it has also been associated with a yellowing and stunting disease of coriander (Tomlinson, 1969) and a golden-yellow chlorosis and necrotic spotting of parsley (Frowd & Tomlinson, 1970).
Geographical Distribution
Celery mosaic virus has been reported from several western states in USA, and also from Florida (Purcifull & Shepard, 1967). Viruses with similar properties have been reported from Germany (Brandes & Luisoni, 1966) and France (Marchoux et al., 1969) and isolates serologically identical to those from USA have been obtained from celery, coriander and parsley in Britain (Walkey, Tomlinson & Frowd, 1970).
Host Range and Symptomatology
Seems to infect only certain members of the family Umbelliferae (Severin & Freitag, 1938; Tomlinson & Carter, 1970).
- Diagnostic species
- Apium graveolens
var. dulce (celery). Green to light green mottling, and malformation of the leaflets. In chronic stages of disease, leaflets become narrowed, cupped and twisted (Fig. 1). Early infection results in pronounced overall stunting and a divergence from the normal upright growth of the petioles (Severin & Freitag, 1938). - Conium maculatum (poison hemlock). Systemic mottle and leaf distortion
(Walkey, 1970). Immune to some strains (Sutabutra, 1968).
- Pastinaca sativa (parsnip). Immune to common strain but susceptible to crinkle leaf strain (Freitag & Severin, 1945).
- Daucus carota var. sativa (carrot). Chlorotic spotting on young leaves of chronically infected plants. Two cultivars, Western Red and Early Nantes, were not infected by mechanical inoculation with British isolates (Walkey et al., 1970).
- Pastinaca sativa (parsnip). Immune to common strain but susceptible to crinkle leaf strain (Freitag & Severin, 1945).
- Propagation species
- A. graveolens
cv. Utah 10B is useful for maintaining the virus and as a source of virus for purification.- Assay species
- No local lesion host known. Celery has been used as a systemic assay host.
Strains
Few strains have been distinguished. A crinkle-leaf strain (Freitag & Severin, 1945) was differentiated on the basis of symptomatology and host range. Strains that are related but serologically distinguishable have been isolated from parsley and poison hemlock in California, USA (Sutabutra, 1968). A virus with long flexuous particles isolated from carrot in Japan was said to be celery mosaic virus, but serological tests were not made and its host range included Nicotiana clevelandii, Chenopodium amaranticolor and C. quinoa in addition to umbelliferous species (Iwaki & Komuro, 1970).
Transmission by Vectors
Transmitted in a non-persistent manner by several aphid spp. Myzus persicae is an efficient experimental vector if starved for 2-6 hr before the acquisition feed. The root-feeding aphids, Aphis middletonii and A. ferruginea-striata also readily transmit; other vector species are A. apigraveolens, A. apii, A. gossypii, A. rumicis, Cavariella capreae, Myzus circumflexus, M. convolvuli and Rhopalosiphum melliferum (Severin & Freitag, 1938), and Cavariella aegopodii and C. pastinacae (Tomlinson & Carter, 1970).
Transmission through Seed
None reported.
Transmission by Dodder
None reported.
Serology
Strongly immunogenic. Useful antisera were produced in rabbits by intramuscular injections of virus emulsified with Freund’s incomplete adjuvant. In tube tests with purified virus, antisera form flocculent precipitates; in double-diffusion tests in agar gel using virus degraded by treatment with detergent, a single straight band of precipitate forms (Shepard & Grogan, 1967b).
Relationships
Morphologically similar to members of the potato virus Y group (Brandes & Wetter, 1959) but serological relationship to recognised members of this group has not been demonstrated. No cross-protection studies have been reported.
Stability in Sap
Inactivated in vitro after 6 days at room temperature. Dilution end-points range from 10-2 to 10-3 and infectivity is lost after heating at 55-60°C for 10 min.
Purification
A procedure for virus concentration and partial purification from celery (Shepard & Grogan, 1967a) involves extraction in 0.5 M borate buffer pH 8.7 and clarification with chloroform, followed by differential and rate zonal density gradient centrifugation.
Properties of Particles
No information.
Particle Structure
Virus particles in leaf dip preparations are flexuous filaments with a normal length of 784 nm (Fig. 2). Particles negatively stained with phosphotungstate show hollow cores.
Particle Composition
Not determined.
Relations with Cells and Tissues
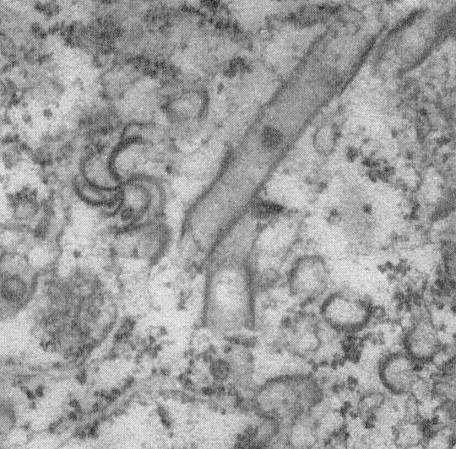
Pinwheel and tubular inclusions (Fig. 3) are present in infected cells (Purcifull & Shepard, 1967).
Notes
In California, USA, the disease in celery has been controlled successfully by the enforcement of a celery-free period which eliminates the principal natural source of virus inoculum (Milbrath, 1948). In Britain, the virus has been isolated from naturally infected wild hemlock and possibly occurs in other weed hosts (Walkey, 1970). Thus a celery-free period may not prove effective for control there.
Figures
References list for DPV: Celery mosaic virus (50)
- Brandes & Luisoni, Phytopath. Z. 57: 277, 1966.
- Brandes & Wetter, Virology 8: 99, 1959.
- Freitag & Severin, Hilgardia 16: 361, 1945.
- Frowd & Tomlinson, Rep. natn. Veg. Res. Stn for 1969: 108, 1970.
- Iwaki & Komuro, Ann. phytopath. Soc. Japan 36: 36, 1970.
- Marchoux, Navatel, Rougier & Duteil, Annls Phytopath. 1: 227, 1969.
- Milbrath, Bull. Calif. Dep. Agric. 37: 3, 1948.
- Purcifull & Shepard, Pl. Dis. Reptr 51: 502, 1967.
- Severin & Freitag, Hilgardia 11: 493, 1938.
- Shepard & Grogan, Phytopathology 57: 1104, 1967a.
- Shepard & Grogan, Phytopathology 57: 1136, 1967b.
- Sutabutra, Ph.D. Thesis, Univ. Calif., Davis, 1968.
- Tomlinson, Rep. natn. Veg. Res. Stn for 1968: 88, 1969.
- Tomlinson & Carter, Rep. natn. Veg. Res. Stn for 1969: 108, 1970.
- Walkey, Rep. natn. Veg. Res. Stn for 1969: 110, 1970.
- Walkey, Tomlinson & Frowd, Pl. Dis. Reptr 54: 370, 1970.