Details of DPV and References
DPV NO: 4 June 1970
Family: Alphaflexiviridae
Genus: Potexvirus
Species: Potato virus X | Acronym: PVX
There is a more recent description of this virus: DPV 354
Potato virus X
R. Bercks Biologische Bundesanstalt für Land und Forstwirtschaft, Braunschweig, Germany
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Smith (1931).
Selected synonyms
- Potato latent virus (Rev. appl. Mycol. 11: 595)
- Potato mild mosaic virus (Rev. appl. Mycol. 22: 367)
- Solanum virus 1 (Rev. appl. Mycol. 17: 52)
- Potato mild mosaic virus (Rev. appl. Mycol. 22: 367)
An RNA-containing virus with elongated particles, normal length c. 515 nm. Most known host plants are in the Solanaceae. Transmission mainly by contact. Very readily transmitted by inoculation of sap. Widely distributed in potato growing countries.
Main Diseases
Causes mild mosaic of potato (Fig. 5), mosaic and slight stunting of tomato, and mottle or necrotic ring spotting of tobacco.
Geographical Distribution
World-wide in potato growing areas.
Host Range and Symptomatology
Very readily transmissible by inoculation of sap. The host range is mostly limited to the Solanaceae, although some plants in other families, e.g. in the Amaranthaceae and Chenopodiaceae, are susceptible. Strains differ greatly in the symptoms they cause. In potato, the symptoms range from complete latency to severe necrotic streak.
Diagnostic species
- Datura stramonium
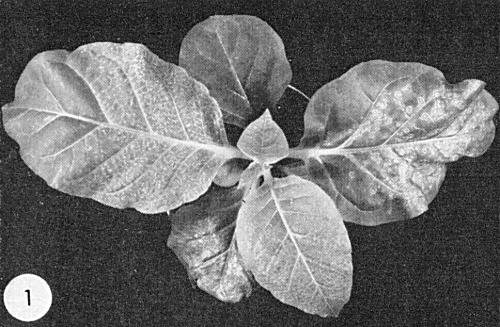
is very susceptible. Most strains give chlorotic rings which later form a mosaic mottle (Fig. 4).- In Nicotiana tabacum (tobacco) and other species of Nicotiana, many strains cause ringspot symptoms (Fig. 1), others cause mottle. Some strains cause no symptoms at high temperatures in glasshouses in the summer.
Propagation species
- Nicotiana tabacum
varieties and other Nicotiana spp.Assay species
- Gomphrena globosa
is a useful local lesion host (Fig. 3). The middle leaves of plants with 8-10 leaves on the main shoot are the most suitable.
Strains
Many minor variants can be distinguished, mainly by the symptoms they give in tobacco. Strains fall into 4 groups on the basis of serology (Matthews, 1949), into 4 other groups on the basis of their infectivity for different potato varieties (Cockerham, 1955; Cockerham & Davidson, 1963) and into a further 3 groups on the basis of thermal inactivation points (Köhler, 1962).
Strains that were originally isolated from potato may lose ability to infect this host after passages on tobacco (Bercks, 1953).
Transmission by Vectors
Transmission occurs mainly by contact. Transmission is reported by the grasshoppers Melanoplus differentialis (Walters, 1952) and Tettigonia viridissima (Schmutterer, 1961). The virus is probably transmitted mechanically on the insect’s mouthparts. Transmission has also been reported by the fungus Synchytrium endobioticum (Nienhaus & Stille, 1965).
Transmission through Seed
None reported.
Transmission by Dodder
Transmission results inconclusive; the virus infects some dodder species (Schmelzer, 1956).
Serology
The virus is strongly immunogenic. It normally reaches high concentrations in host plants and can readily be detected serologically by using the slide precipitin test in which the precipitates are flocculent (flagellar). Agar gel-diffusion tests can be done with virus fragments (Tomlinson & Walkey, 1967).
Relationships
Serologically the strains of potato virus X fall into 4 groups (Matthews, 1949). In plant protection tests, strains may protect against the effects of one another completely, partially or not at all.
There is a distant serological relationship with the following viruses of the same or similar normal lengths: white clover mosaic, hydrangea ringspot, cactus X and clover yellow mosaic. Because these viruses also possess other common properties they can be grouped together to form the potato virus X group (Brandes & Bercks, 1965).
Stability in Sap
In tobacco sap, the thermal inactivation point (10 min) is between 68 and 76°C depending on the strain, the dilution end-point is between 10-5 and 10-6, and infectivity is retained at 20°C for several weeks and in the presence of glycerol for more than 1 year.
Purification
Several methods seem satisfactory. The virus has a tendency to aggregate. To avoid aggregation the following procedure is recommended by Francki & McLean (1968): the plant sap is clarified by adsorption with charcoal and DEAE-cellulose and then filtered through Celite, the virus concentrated by ultracentrifugation, and the pellet resuspended in distilled water. The suspension is emulsified with chloroform, and the virus precipitated from the aqueous phase by ultracentrifugation. Chloroform treatment and ultracentrifugation are repeated and the virus is suspended in distilled water. In other published methods, which disregard the possibility of aggregation, the plant sap is often clarified with organic solvents; for example Pfankuch & Kausche (1938) diluted the sap with an equal volume of water and stirred it for 20 min with 1/7 volume of chloroform. For investigating the properties of the particles, clarification procedures should be done rapidly because prolonged contact with crude plant sap causes degradation of the protein subunits (Koenig et al., 1970).
Properties of Particles
Sedimentation coefficient (s20,w) at infinite dilution: 117.7 S.
Molecular weight (daltons): 35 x 106 (Reichmann, 1959).
Isoelectric point: pH 4.4.
Partial specific volume: 0.73 cm3/g.
Electrophoretic mobility: -4 x 10-6/cm2 sec-1 volt-1 in 0.06 M phosphate buffer at pH 7.0 (Bawden & Kleczkowski, 1959).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 2.97 (Paul, 1959).
A260/A280: 1.20 (Paul, 1959).
Particle Structure
Particles are flexuous filaments (Fig. 2) with helical substructure of normal length c. 515 nm, diameter c. 13 nm (Brandes, 1964), with cross banding and a central hole of diameter c. 3.4 nm. Pitch of helix 3.4 nm (Varma et al., 1968), about 10 subunits per turn (Wilson & Tollin, 1969).
Particle Composition
RNA: Molecular weight 2.1 x 106, single stranded. Molar percentages of nucleotides: G22; A32; C24; U22. RNA is about 6% of particle weight (Knight, 1963).
Protein: Subunits in rapidly purified preparations have a molecular weight of c. 3.0 x 104; after prolonged contact with crude sap, values of 2.2-2.4 x 104 have been found (Koenig et al., 1970; Miki & Knight, 1968). The amino acid composition of the protein has been reported for different strains by Shaw & Larson (1962), Shaw, Reichmann & Hatt (1962) and Miki & Knight (1968). For the ringspot strain it is (moles percent): ala 13.4; arg 6.3; asx 11.3; glx 9.4; his 1.2; ile 5.5; leu 5.1; lys 6.4; met 4.2; phe 7.3; pro 7.2; ser 6.4; thr 13.8; tyr 1.4; val 6.2 (Shaw, Reichmann & Hatt, 1962).
Relations with Cells and Tissues
During the early phases of infection the virus is found mainly in the palisade cells, less frequently in the epidermis, of Datura stramonium and potato. Particles are distributed diffusely or in densely packed aggregates or in X-bodies. The aggregates may fill the greater part of the cells. Virus particles and spherical inclusion bodies occur in the plastids. The X-bodies, which occur mainly near the nucleus, also contain cellular components, chiefly ribosome like particles, either free or in linear groups from 500 to 1600 nm in length; mitochondria, dictyosomes and areas of endoplasmic reticulum are also present (Kozar & Sheludko, 1969).
Figures
References list for DPV: Potato virus X (4)
- Bawden & Kleczkowski, Virology 7: 375, 1959.
- Bercks, Phytopath. Z. 20: 113, 1953.
- Brandes, Mitt. biol. BundAnst. Ld- u. Forstw. 110, 130 pp., 1964.
- Brandes & Bercks, Adv. Virus Res. 11: 1, 1965.
- Cockerham, Proc. 2nd Conf. Potato Diseases, Lisse-Wageningen, 1954: 89, 1955.
- Cockerham & Davidson, Rep. Scott. Pl. Breed. Stn 1963: 26, 1963.
- Francki & McLean, Aust. J. biol. Sci. 21: 1311, 1968.
- Knight, Protoplasmatologia 4(2): 177 pp., 1963.
- Koenig, Stegemann, Francksen & Paul, Biochim., biophys. Acta 207: 184, 1970.
- Köhler, Phytopath. Z. 44: 189, 1962.
- Kozar & Sheludko, Virology 38: 220, 1969.
- Matthews, Ann. appl. Biol. 36: 460, 1949.
- Miki & Knight, Virology 36: 168, 1968.
- Nienhaus & Stille, Phytopath. Z. 54: 335, 1965.
- Paul, Arch. Mikrobiol. 32: 416, 1959.
- Pfankuch & Kausche, Biochem. Z. 299: 334, 1938.
- Reichmann, Can. J. Chem. 37: 384, 1959.
- Schmelzer, Phytopath. Z. 28: 1, 1956.
- Schmutterer, J. angew. Ent. 47: 277, 1961.
- Shaw & Larson, Phytopathology 52: 170, 1962.
- Shaw, Reichmann & Hatt, Virology 18: 79, 1962.
- Smith, Proc. R. Soc., B 109: 251, 1931.
- Tomlinson & Walkey, Virology 32: 267, 1967.
- Varma, Gibbs, Woods & Finch, J. gen. Virol. 2: 107, 1968.
- Walters, Phytopathology 42: 355, 1952.
- Wilson & Tollin, J. gen. Virol. 5: 151, 1969.