Details of DPV and References
DPV NO: 9 June 1970
Family: Potyviridae
Genus: Potyvirus
Species: Lettuce mosaic virus | Acronym: LMV
There is a more recent description of this virus: DPV 399
Lettuce mosaic virus
J. A. Tomlinson National Vegetable Research Station, Wellesbourne, Warwickshire, England
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Described by Jagger (1921).
- Selected synonyms:
- Lactuca
virus 1 (Rev. appl. Mycol. 17: 52) - Marmor lactucae (Rev. appl. Mycol. 28: 514)
- A virus with flexuous filamentous particles approximately 750 x 13 nm. It is sap-transmissible to a wide range of species, is seed-borne in lettuce and is transmitted by several aphid species in the non-persistent manner. World-wide in distribution.
Main Diseases
Causes various mosaic and mottle symptoms in almost all types of lettuce (Lactuca sativa and L. sativa var. longifolia).
Geographical Distribution
World-wide. The disease is widespread in the USA, especially California, and in Europe.
Host Range and Symptomatology
Host range is wide (Costa & Duffus, 1958). Susceptible species occur in 20 genera (9 genera of Compositae) in 10 families. Transmissible by inoculation with sap from young infected plants, but transmission with sap from old leaves may be difficult.
- Diagnostic species
- Lactuca sativa
(lettuce). Symptoms are variable but usually consist of vein clearing and yellow mottling sometimes with veinal necrosis (conspicuous in the flowering plant) and bronzing. Plants fail to ‘heart’ and inner leaves remain dwarfed and rosetted (Fig. 1). - Chenopodium amaranticolor. Pale green or chlorotic local lesions
(usually with reddish margins) after 8-10 days (Fig. 2, left). Systemic
yellow veinal flecks or yellow netting of the younger leaves especially in
winter (Fig. 2, right).
- C. quinoa. More sensitive than C. amaranticolor: local lesions more numerous but without reddish margins. Conspicuous, systemic yellow vein-net symptoms with twisting and stunting of apical leaves (Fig. 3).
- Gomphrena globosa. Whitish, local necrotic dots (4-7 days) enlarging into red-rimmed lesions.
- C. quinoa. More sensitive than C. amaranticolor: local lesions more numerous but without reddish margins. Conspicuous, systemic yellow vein-net symptoms with twisting and stunting of apical leaves (Fig. 3).
- Propagation species
- Lettuce plants 10-15 days after inoculation are suitable sources of virus
for purification. Systemically infected safflower (Carthamus tinctorius) is also
reported (Klisiewicz, 1965) to be a good source.
- Chenopodium quinoa can be used for maintaining cultures.
- Chenopodium quinoa can be used for maintaining cultures.
- Assay species
- Chenopodium amaranticolor, C. quinoa
and Gomphrena globosa are suitable local lesion hosts.
Strains
McLean & Kinsey (1962, 1963) differentiated four Californian strains by symptoms in lettuce and pea.
Transmission by Vectors
Transmissible by several species of aphid (Dickson & Laird, 1959; Kennedy, Day & Eastop, 1962) notably Myzus persicae, Macrosiphum euphorbiae and Acyrthosiphon scariolae barri. Non-vectors include Nasonovia ribisnigri. All instars of M. persicae transmit but alates are less efficient than apterae. Transmission efficiency increases with increasing periods of fasting (5-240 min), but decreases with increasing acquisition access time from 5 to 120 min (Sylvester, 1955).
Transmission through Seed
The virus is seed-borne in lettuce (Newhall, 1923), some 3-10% of the seed giving rise to infected seedlings, depending on the time of infection of the mother plant (Couch, 1955) and the variety (Grogan, Welch & Bardin, 1952). Transmission occurs through both pollen and ovules of infected plants (Ryder, 1964). Seed transmission also occurs in Lactuca serriola (Van Hoof, 1959) but not in the lettuce variety Cheshunt Early Giant (Kassanis, 1947). Seed transmission was reported in Senecio vulgaris (Ainsworth & Ogilvie, 1939; Kemper, 1962), but was unconfirmed by Fry (1952).
Seed transmission is probably the major factor in the spread of the disease (Broadbent, Tinsley, Buddin & Roberts, 1951; Grogan et al., 1952; Tomlinson, 1962). Spread occurs (a) from seedlings infected through the seed and (b) from neighbouring infected lettuce. The disease can be controlled by ensuring that the crop is isolated from external sources of virus and that less than 0.1% of the seed carries the virus (Zink, Grogan & Welch, 1956; Tomlinson, 1962). Even where adjacent crops are infected, use of mosaic-free seed provides some control (Tomlinson, 1962).
Transmission by Dodder
No reports.
Serology
An antiserum prepared by intravenous injection of a purified preparation had a titre of 1/256 and gave a flagellar-type precipitate in precipitin tube tests. Agglutination tests with crude sap were about 90% reliable (Tomlinson, 1964).
Relationships
The virus belongs to the potato virus Y group. According to Brandes & Bercks (1965), lettuce mosaic virus may be distantly serologically related to turnip mosaic and sugarcane mosaic viruses.
In plant protection tests, some strains of the virus protected lettuce against other more virulent strains (McLean & Kinsey, 1962, 1963). Lettuce mosaic virus did not protect lettuce against dandelion yellow mosaic virus (Kassanis, 1947).
Stability in Sap
In lettuce sap, the thermal inactivation point (10 min) is 55-60°C, dilution end-point 10-1-10-2 and infectivity is retained at 20°C for 1-2 days. Infectivity is considerably stabilized by adding 0.5% sodium sulphite (Ainsworth & Ogilvie, 1939) or 0.1% thioglycollic acid (Tomlinson, 1964).
Purification
Tomlinson (1964). Extract infected lettuce leaves at pH 7.5 in 0.5 M sodium borate containing 0.1% thioglycollic acid. Filter and add n-butanol to 8.5% (v/v). Centrifuge twice at low speed. Sediment and clarify by high and low speed centrifugation, resuspending the pellets obtained at high speed in 0.05 M borate (pH 7.5). Do all steps at 3°C. Preparations infective to 10-3.
Properties of Particles
No reports.
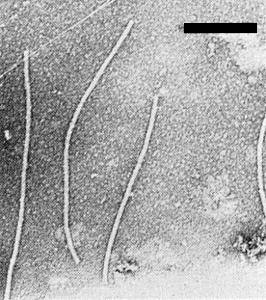
Particle Structure
Particles are flexuous filaments approximately 750 x 13 nm (Fig. 4). They do not appear to be penetrated by phosphotungstate.
Particle Composition
No reports.
Relations with Cells and Tissues
No reports.
Notes
Lettuce mosaic virus may sometimes be found in lettuce together with cucumber mosaic virus and both viruses may be simultaneously transmitted in sap to Chenopodium amaranticolor or C. quinoa. Unlike cucumber mosaic virus, which causes only local lesions in the inoculated leaves (2-3 days), lettuce mosaic virus infects both these hosts systemically.
Acknowledgements
Photographs: courtesy of National Vegetable Research Station.
Figures
References list for DPV: Lettuce mosaic virus (9)
- Ainsworth & Ogilvie, Ann. appl. Biol. 26: 279, 1939.
- Brandes & Bercks, Adv. Virus Res. 11: 1, 1965.
- Broadbent, Tinsley, Buddin & Roberts, Ann. appl. Biol. 38: 689, 1951.
- Costa & Duffus, Pl. Dis. Reptr 42: 583, 1958.
- Couch, Phytopathology 45: 63, 1955.
- Dickson & Laird, J. econ. Ent. 52: 440, 1959.
- Fry, N.Z. Jl Sci. Technol. Sect. A 33: 52, 1952.
- Grogan, Welch & Bardin Phytopathology 42: 573, 1952.
- Jagger, J. agric. Res. 20: 739, 1921.
- Kassanis, Ann. appl. Biol. 34: 412, 1947.
- Kemper, Z. PflKrankh. PflPath. PflSchutz 69: 653, 1962.
- Kennedy, Day & Eastop, A conspectus of aphids as vectors of plant viruses, London, Commonwealth Institute of Entomology, 1962.
- Klisiewicz, Pl. Dis. Reptr 49: 541, 1965.
- McLean & Kinsey, Phytopathology 52: 403, 1962.
- McLean & Kinsey, Pl. Dis. Reptr 47: 474, 1963.
- Newhall, Phytopathology 13: 104, 1923.
- Ryder, Pl. Dis. Reptr 48: 522, 1964.
- Sylvester, Phytopathology 45: 357, 1955.
- Tomlinson, Pl. Path. 11: 61, 1962.
- Tomlinson, Ann. appl. Biol. 53: 95, 1964.
- Van Hoof, Tijdschr. PlZiekt. 65: 44, 1959.
- Zink, Grogan & Welch, Phytopathology 46: 662, 1956.