Details of DPV and References
DPV NO: 6 June 1970
Family: Secoviridae
Genus: Nepovirus
Species: Raspberry ringspot virus | Acronym: RpRSV
There is a more recent description of this virus: DPV 198
Raspberry ringspot virus
A. F. Murant Scottish Horticultural Research Institute, Invergowrie, Dundee, Scotland
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by
Cadman (1956) and
Harrison (1958).
- Selected synonyms
- Raspberry Scottish leaf curl virus (Rev. appl. Mycol. 32: 387)
- Red currant ringspot virus (Rev. appl. Mycol. 31: 245)
- Red currant ringspot virus (Rev. appl. Mycol. 31: 245)
- An RNA-containing virus with isometric particles about 30 nm in diameter occurring in Europe. It is readily sap-transmissible, has a wide host range, infects the seed of many host plants, and is transmitted by nematodes (Longidorus spp.).
Main Diseases
Causes ringspot diseases of raspberry and strawberry, spoonleaf of redcurrant and, in association with viruses of the prunus necrotic ringspot type, is one of the causes of rasp-leaf symptoms in cherry. It occurs in many other plants, including grapevine and Narcissus.
Geographical Distribution
Europe.
Host Range and Symptomatology
Occurs naturally in many species of wild and cultivated monocotyledonous and dicotyledonous plants. Species in more than 14 dicotyledonous families are susceptible. Infects nearly all commonly used herbaceous test plants.
Diagnostic species
- Chenopodium amaranticolor. Chlorotic or necrotic local lesions
(Fig. 1);
not systemic.
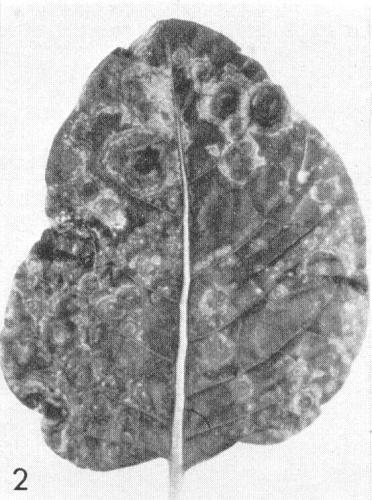
- C. quinoa. Chlorotic or necrotic local lesions; systemic chlorotic mottle or necrosis (Fig. 4).
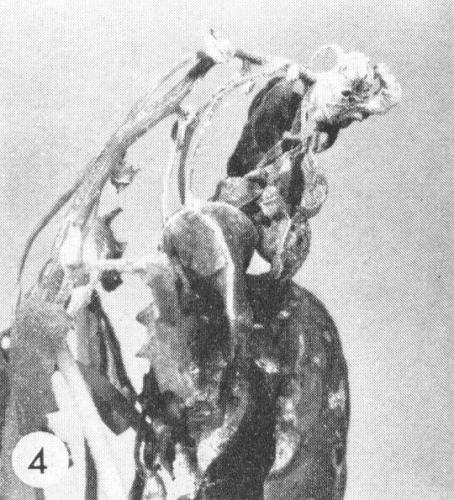
- Nicotiana rustica. Local chlorotic or necrotic spots or rings; systemic rings, spots and line-patterns with variable amounts of necrosis (Fig. 2). Leaves produced later appear normal but contain virus.
- N. tabacum cv. White Burley (tobacco). Chlorotic local lesions; scattered systemic chlorotic spots and rings.
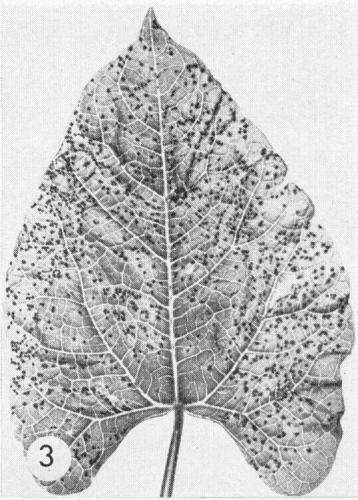
- Phaseolus vulgaris cv. The Prince (French bean). In winter in the UK, dark brown necrotic local lesions 0.5 mm diameter (Fig. 3); in summer chlorotic lesions or none; systemic chlorotic mottle, necrosis and distortion.
- Petunia hybrida. Local chlorotic lesions; systemic veinal chlorosis or necrosis or line-patterns (Fig. 5). Leaves produced subsequently are symptomless but contain virus.
- C. quinoa. Chlorotic or necrotic local lesions; systemic chlorotic mottle or necrosis (Fig. 4).
- Propagation species
- Nicotiana rustica is useful for maintaining cultures, N. clevelandii and Petunia hybrida
are good sources of virus for purification.
- Assay species
- Chenopodium amaranticolor is the most reliable local lesion host. C. quinoa and Spinacia oleracea (spinach) are convenient ‘bait plants’ in nematode transmission experiments.
Strains
Many minor variants occur. Three important strains are:
The Scottish strain
(Cadman, 1956;
Harrison, 1958).
The type strain.
The English strain
(Harrison, 1958;
Cadman, 1960),
which differs from the Scottish strain
serologically and in vector relationships (see below).
The Lloyd George yellow blotch strain (Murant, Taylor & Chambers, 1968), which infects varieties of raspberry immune to the Scottish strain but is serologically similar to it.
Transmission by Vectors
Transmitted by free-living soil-inhabiting nematodes (Longidorus spp.). The Scottish strain and strains closely related serologically are transmitted most efficiently by L. elongatus, the English strain by L. macrosoma (Taylor, 1962; Harrison, 1964; Taylor & Murant, 1969). Larvae and adults of L. elongatus both transmit, but the adult does not pass the virus to its progeny, nor is the virus retained when the nematode moults. L. elongatus kept in fallow soil retains infectivity up to about 9 weeks. Virus-like particles were associated with the stylet guiding sheath of L. elongatus which had fed on plants infected with raspberry ringspot virus (Taylor & Robertson, 1969).
Transmission through Seed
Probably occurs in most host plants. Reported in at least six species in five plant families. In some hosts more than 50% of progeny seedlings are infected. Many plants infected through seed show no symptoms. Virus was transmitted to seed of raspberry and strawberry from either male or female parent, but plants pollinated with virus-carrying pollen did not become infected (Lister & Murant, 1967). Besides aiding dissemination of the virus, infection of the seed seems to provide an important means of survival of the virus in soils (Murant & Lister, 1967).
Transmission by Dodder
No information.
Serology
Antisera with titres of 1/500 are readily obtained. Precipitin tests in tubes, microprecipitin tests or gel-diffusion tests in 1% agar are satisfactory. The virus produces a single band of precipitate in gel-diffusion tests.
Relationships
The Scottish strain is closely serologically related to a strain causing spoonleaf disease of redcurrant in the Netherlands (Harrison, 1961; Maat, Van der Meer & Pfaeltzer, 1962) but most antisera readily distinguish these strains from those found in England and Germany (Cadman, 1960).
Plant-protection between virulent isolates of serologically different strains is usually complete (Harrison, 1958) but avirulent isolates may not protect plants from infection with virulent isolates, even when they are closely serologically related (Murant, Taylor & Chambers, 1968).
Raspberry ringspot virus resembles in many properties other nepoviruses (arabis mosaic, grapevine fanleaf, strawberry latent ringspot, tobacco ringspot, tomato black ring and tomato ringspot), but is unrelated to them serologically.
Stability in Sap
In Nicotiana rustica sap the virus loses infectivity after 10 min at 65-70°C, storage at room temperature for 2-3 weeks, or dilution to 10-3-10-4. The lesion number decreases with dilution by more than the dilution factor (Harrison, 1958).
Purification
A modification of Steere’s butanol/chloroform method is useful (Harrison & Nixon, 1960).
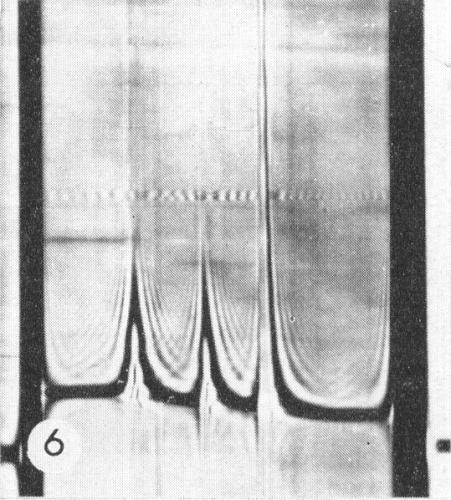
Properties of Particles
The particles are all the same size but sediment as three components
(Fig. 6),
apparently
empty protein shells (T) and two kinds of nucleoprotein with different amounts of RNA
(M and B).
Sedimentation coefficients (s20,w) (svedbergs): about 50 (T), 91 (M),
125 (B).
A260/A280: 1.62 (M), 1.78 (B).
Particle Structure
Isometric, about 30 nm in diameter with a 5- or 6-sided angular outline (Harrison & Nixon, 1960). Electron micrographs show some particles completely, some partially, and some not penetrated by phosphotungstate (Fig. 7). These particles possibly correspond respectively to the T, M and B components (Debrot, 1964; Murant, Taylor & Chambers, 1968). Detailed structure of the particle is unknown.
Particle Composition
RNA constitutes about 29% (M) and 43% (B) of the particle weight (estimated from the sedimentation coefficients).
Relations with Cells and Tissues
Inclusion bodies were observed by light microscopy in hair cells of infected Petunia hybrida (Bujas & Milicic, 1969).
Notes
As with other nematode-borne viruses, plants infected with raspberry ringspot virus are patchily distributed in crops (Fig. 8), reflecting the distribution of the vectors, which migrate slowly in soils. Some strains often occur in soils together with strains of tomato black ring virus, with which they share the same nematode vector (Longidorus elongatus). These two viruses are serologically unrelated. They can be distinguished from each other by their reactions in Chenopodium amaranticolor. In general, however, these and other viruses of the nepovirus group cannot be reliably identified by host range or symptomatology; serological tests are essential.
Figures
References list for DPV: Raspberry ringspot virus (6)
- Bujas & Milicic, Zentbl. Bakt. ParasitKde, Abt.II 123: 209, 1969.
- Cadman, J. hort. Sci. 31: 111, 1956.
- Cadman, Virology 11: 653, 1960.
- Debrot, Ann. appl. Biol. 54: 183, 1964.
- Harrison, Ann. appl. Biol. 46: 571, 1958.
- Harrison, Tijdschr. PlZiekt. 67: 562, 1961.
- Harrison, Virology 22: 544, 1964.
- Harrison & Nixon, Virology 12: 104, 1960.
- Lister & Murant, Ann. appl. Biol. 59: 49, 1967.
- Maat, Van der Meer & Pfaeltzer, Tijdschr. PlZiekt. 68: 120, 1962.
- Murant & Lister, Ann. appl. Biol. 59: 63, 1967.
- Murant, Taylor & Chambers, Ann. appl. Biol. 61: 175, 1968.
- Taylor, Virology 17: 493, 1962.
- Taylor & Murant, Ann. appl. Biol. 64: 43, 1969.
- Taylor & Robertson, Ann. appl. Biol. 64: 233, 1969.