Details of DPV and References
DPV NO: 203 July 1979
Family: Secoviridae
Genus: Comovirus
Species: Andean potato mottle virus | Acronym: APMoV
Andean potato mottle virus
C. E. Fribourg Universidad Nacional Agraria, Apartado 456, Lima, Peru
R. A. C. Jones International Potato Center, Apartado 5969, Lima, Peru
R. Koenig Biologische Bundesanstalt, D33, Braunschweig, Germany
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Fribourg, Jones & Koenig (1977a).
A virus with isometric, RNA-containing particles about 26 nm in diameter. It has a narrow host range, is readily transmissible by manual inoculation and is widespread in potato crops in the Andean region of South America.
Main Diseases
In most potato cultivars, primary and secondary symptoms are mild or severe mottles (Fig. 1). Sensitive cultivars may react with initial top necrosis, leaf deformation and/or stunting (Fig. 2, Fig. 3). Plants growing under cool conditions may develop yellow spotting, blotching or more generalized yellowing on leaves.
Geographical Distribution
Andean region of South America.
Host Range and Symptomatology
Readily transmissible by inoculation of sap. Host range mostly limited to the Solanaceae.
-
Diagnostic species
- Chenopodium amaranticolor
and C. quinoa. Immune. - Lycopersicon chilense and Nicandra physalodes. Systemic vein-
clearing, interveinal mosaic and chlorotic spotting
(Fig. 5).
- Nicotiana bigelovii. Systemic mosaic consisting of dark green blotches. Tip leaves may develop necrotic areas and holes (Fig. 4).
- N. clevelandii. Systemic mosaic.
- Nicotiana bigelovii. Systemic mosaic consisting of dark green blotches. Tip leaves may develop necrotic areas and holes (Fig. 4).
-
Propagation species
- Nicotiana bigelovii
and N. clevelandii are suitable species for maintaining cultures and are good sources of virus for purification.Assay species
- The virus may be assayed in N. bigelovii and N. clevelandii by determining the proportion of plants that become systemically infected. No host suitable for local lesion assay is known.
Strains
Two distinct strains are recognized:
Strain Lm of Fribourg, Jones & Koenig (1977a). The type strain.
Strain H of Salazar & Harrison (1978).
These strains are serologically distinguishable but probably cannot be reliably distinguished by symptoms on host plants.
Transmission by Vectors
Transmission occurs by contact. No vector species is known, but possibly the virus, like other comoviruses, is transmitted by beetles. Epitrix sp. was not a vector (Fribourg, Jones & Koenig, 1977a).
Transmission through Seed
The virus was not transmitted through seed of Nicotiana bigelovii or of potato cv. Renacimiento (Fribourg, Jones & Koenig, 1978).
Serology
The virus is very immunogenic; rabbit antiserum with a titre of 1/4096 was readily obtained (Fribourg, Jones & Koenig, 1977a). Gel diffusion tests in 1% agarose are convenient for detecting the virus in crude sap; one line of precipitate is produced. The latex flocculation and ELISA techniques are also useful, for example to detect the virus in potato tubers (Koenig & Bode, 1978).
Relationships
Strains Lm and H are serologically related but distinguishable, producing spurs when compared in gel-diffusion tests using antiserum to either (Salazar & Harrison, 1978). The virus is serologically distantly related to cowpea mosaic, bean pod mottle, and quail pea mosaic viruses, and is therefore placed in the comovirus group (Fribourg, Jones & Koenig, 1977a). It also shares many properties with members of this group that are not related to it serologically.
Stability in Sap
In Nicotiana bigelovii sap, the thermal inactivation point (10 min) is 65-70°C, the dilution end-point is more than 10-6 and infectivity is retained at 20°C for at least 4 weeks.
Purification
Steere’s butanol/chloroform method or the bentonite method of Dunn & Hitchborn (1965) are both suitable (Fribourg, Jones & Koenig, 1977a).
Properties of Particles
The particles are all the same size but sediment as three components, empty protein shells (T) and two kinds of nucleoprotein with different amounts of RNA (M and B).
Sedimentation coefficients (s°20,w) (svedbergs): 53 (T), 93 (M), 112 (B) (Salazar & Harrison, 1978).
A260/A280 of unfractionated virus: 1.5 (Fribourg, Jones & Koenig, 1977a).
Buoyant density in CsCl (g/ml): 1.41(M), 1.46 (B) (Salazar & Harrison, 1978).
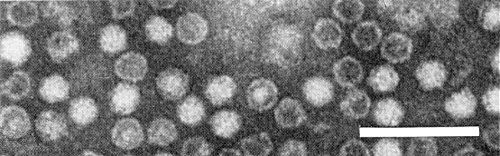
Particle Structure
Particles are isometric, c. 25-27 nm in diameter with somewhat hexagonal outlines. Electron micrographs show particles, some completely, some partially and some not penetrated by negative stain (Fig. 6). Detailed structure of the particles is not known.
Particle Composition
Nucleic acid: RNA, single-stranded, comprising about 27% and 34% respectively of the weight of M and B particles, estimated by assuming that the particles contain 60 molecules of each polypeptide species. The two RNA species have M. Wt of 1.43 x 106 and 2.0 x 106, estimated by polyacrylamide gel electrophoresis under denaturing conditions; M particles contain only the smaller species and B particles only the larger (Salazar & Harrison, 1978).
Protein: Particles contain c. 3.8 x 106 daltons of protein. Two polypeptide species of M. Wt 20,800 and 40,100 (Fribourg, Jones & Koenig, 1977a) or 22,100 and 41,800 (Salazar & Harrison, 1978).
Relations with Cells and Tissues
No information.
Notes
Andean potato mottle virus and the tymovirus, Andean potato latent virus, are contact-transmitted viruses with similar particle size, shape, and properties in sap, and both are common in potato crops in the Andean region of South America. However, they can be readily distinguished by differences in host range and symptomatology in indicator hosts: Andean potato latent virus infects species in the Chenopodiaceae and Cucurbitaceae, and produces more severe symptoms in N. bigelovii and N. clevelandii but less severe effects on potato cultivars. Moreover, only Andean potato latent virus is transmitted by the flea beetle Epitrix sp. and the two viruses are serologically unrelated (Fribourg, Jones & Koenig, 1977a, 1977b; Jones & Fribourg, 1977, 1978).
Figures
References list for DPV: Andean potato mottle virus (203)
- Dunn & Hitchborn, Virology 25: 171, 1965.
- Fribourg, Jones & Koenig, Phytopathology 67: 969, 1977a.
- Fribourg, Jones & Koenig, Ann. appl. Biol. 86: 373, 1977b.
- Fribourg, Jones & Koenig, Fitopatología 13: 28,1978.
- Jones & Fribourg, Ann. appl. Biol. 86: 123, 1977.
- Jones & Fribourg, Potato Res. 21: 121, 1978.
- Koenig & Bode, Phytopath. Z. 92: 275, 1978.
- Salazar & Harrison, J. gen. Virol. 39: 171, 1978.