Details of DPV and References
DPV NO: 247 July 1982
Family: Alphaflexiviridae
Genus: Potexvirus
Species: Viola mottle virus | Acronym: VMoV
Viola mottle virus
V. Lisa Istituto di Fitovirologia applicata del C.N.R., Torino, Italy
G. Boccardo Istituto di Fitovirologia applicata del C.N.R., Torino, Italy
R. G. Milne Istituto di Fitovirologia applicata del C.N.R., Torino, Italy
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Lisa & Dellavalle (1977).
A virus with filamentous particles c. 480 nm long, consisting of one type of coat protein and one molecule of single-stranded RNA. Easily transmitted mechanically but no vector is known; the virus is spread mainly by vegetative propagation of the host.
Main Diseases
Occurs naturally in Viola odorata, causing reduction in growth, mottle on leaves (Fig. 1) and white stripes on the petals (Fig. 2).
Geographical Distribution
Reported from Northern Italy only. See also under Notes.
Host Range and Symptomatology
Symptoms on V. odorata are described under Main Disease. The virus is easily sap-transmitted; it infected 20 of 36 species in 10 of 13 dicotyledonous families (Aizoaceae, Amaranthaceae, Caryophyllaceae, Chenopodiaceae, Compositae, Leguminosae, Primulaceae, Scrophulariaceae, Solanaceae, Violaceae); in most species the virus caused latent infection only (Lisa & Dellavalle, 1977).
Diagnostic species
- Amaranthus caudatus.
Necrotic local lesions; systemic mottle.- Chenopodium amaranticolor. Chlorotic local lesions; systemic mosaic
(Fig. 3).
- Gomphrena globosa. Necrotic local lesions; systemic infection, usually latent.
- Nicotiana clevelandii. Latent infection in inoculated leaves; no systemic infection.
- Gomphrena globosa. Necrotic local lesions; systemic infection, usually latent.
Propagation species
- The virus is best maintained in Chenopodium quinoa; inoculated and systemically infected leaves of this host are good sources of virus for purification.
Assay species
- Chenopodium quinoa
and Gomphrena globosa consistently give good local lesions.
Strains
None reported.
Transmission by Vectors
No reports.
Transmission through Seed
No reports.
Serology
The virus is a good immunogen; positive reactions at antiserum dilutions up to 1/2048 were observed in slide precipitin tests (Lisa & Dellavalle, 1977).
Relationships
Distant serological relationships were detected to cactus virus X (strain BS) and hydrangea ringspot virus (Lisa & Dellavalle, 1977), foxtail mosaic virus (Short, 1981), nerine virus X (Agapanthus isolate) (Phillips & Brunt, 1980) and tulip virus X (Mowat, 1979). Viola mottle virus did not react with antisera to strains CC10 and K11 of cactus virus X, or to clover yellow mosaic, narcissus mosaic, papaya mosaic, pepino mosaic or white clover mosaic viruses. Antisera to viola mottle virus did not react with cymbidium mosaic or potato X viruses (Lisa & Dellavalle, 1977, and unpublished data).
Stability in Sap
Infectivity was retained in Chenopodium quinoa sap after heating for 10 min at 95°C but not 99°C (although most of it was lost at about 80°C); after dilution with distilled water up to 10-9 but not 10-10, and after 8 months storage at room temperature (Lisa & Dellavalle, 1977).
Purification
Homogenize inoculated and systemically infected Chenopodium quinoa leaves, 10-12 days after inoculation, in 0.5 M borate buffer, pH 8.6, containing 0.01 M sodium diethyldithiocarbamate, 0.005 M sodium ethylenediamine-tetraacetate and 0.02 M sodium sulphite. Emulsify the extract with chloroform (or with a 1:1 mixture of chloroform and n-butanol) and clarify by low speed centrifugation. Sediment the virus from the aqueous phase by high speed centrifugation (78,000 g for 90 min). Resuspend the virus in 0.002 M phosphate buffer, pH 7, and further purify in linear 10-50% sucrose gradients (Beckman SW 25.2 rotor, 25,000 rev/min, 90 min) (Lisa & Dellavalle, 1977).
The virus has also been purified by Richardson, Tollin & Bancroft (1981) by a method originally devised by Bancroft, Abou Haidar & Erickson (1979) for clover yellow mosaic virus; this involves extraction in 0.02 M sodium borate buffer, pH 8.2, clarification with 0.5% Triton X-100 and further purification by ultracentrifugation through sucrose cushions and a cycle of differential centrifugation.
Properties of Particles
A260/A280: 1.23; Amax(264)/Amin(246): 1.30 (corrected for light-scattering; Lisa & Dellavalle, 1977).
Buoyant density in CsCl at 10°C: 1.31 g/cm3 (V. Lisa & G. Dellavalle, unpublished data).
Particle Structure
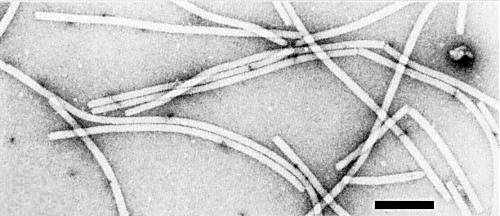
Flexuous filaments (Fig. 7) of modal length ranging from 478 nm in phosphotungstate to 495 nm in uranyl acetate (Lisa & Dellavalle, 1977). Diameter 13-13.5 nm, in uranyl acetate or formate. Fine structure is typical of potexviruses, i.e., both cross-banding (pitch about 3.6 nm) and longitudinal files of subunits are visible, including a faint indication of the axial canal (Fig. 8) (R. G. Milne, unpublished data).
By optical diffraction, the pitch of the helix appeared to be 3.5 (±0.05) nm, with 8.83 subunits per turn and a true repeat in 6 turns (Richardson et al., 1981). These structural parameters of the virus particles are essentially the same as those for papaya mosaic (Tollin et al., 1979), clover yellow mosaic (Tollin et al., 1981), narcissus mosaic (Bancroft, Hills & Richardson, 1980), potato X (Tollin, Wilson & Bancroft, 1980), cymbidium mosaic, nerine X, barrel cactus and foxtail mosaic viruses (Richardson et al., 1981).
Particle Composition
Nucleic acid: RNA, single-stranded (G 22.8; A 26.4; C 30.3; U 20.5), with an apparent M. Wt of 2.2-2.6 x 106 in slab gel electrophoresis (Fig. 6) under partially denaturing conditions as described by Gould (1981) (2.6% acrylamide, 0.17% methylene bisacrylamide, 7 M urea, 90 mM Tris-borate, 3 mM sodium ethylenediamine-tetraacetate, pH 8.3) (G. Boccardo, unpublished data).
Protein: Purified virus particles resuspended in 6 M urea, 2% (w/v) 2-mercaptoethanol, 1% (w/v) sodium dodecyl sulphate and 0.001% (w/v) bromophenol blue in 0.125 M Tris-HCl, pH 6.8, yielded electrophoretically homogeneous subunits (Fig. 5) of M. Wt 2.1 x 104 (mean of 14 determinations, s.e. = 0.1 x 104) (G. Boccardo, unpublished data).
M. N. Short (personal communication) calculated a M. Wt of 21,473 based on 204 amino acid residues (lys 5, his 1, arg 9, asp 14, thr 25, ser 17, glu 18, pro 17, gly 14, ala 27, half cys 2, val 8, met 1, ile 7, leu 24, tyr 3, phe 10, trp 2).
Relations with Cells and Tissues
Masses of virus particles occur in the cytoplasm of parenchyma cells of C. quinoa (Fig. 4). No structures such as beaded sheets (Lesemann & Koenig, 1977) are found in infected cells. There are no obvious alterations of the cell organelles, except when the cells are killed (R. G. Milne, unpublished data).
Notes
Cucumber mosaic virus is also common in V. odorata but can be easily distinguished from viola mottle virus by serological assays, electron microscopy or by differential reactions of test plants: viola mottle virus does not infect most species of Solanaceae that are susceptible to cucumber mosaic virus, and gives systemic infection of species of Chenopodiaceae, in which cucumber mosaic virus usually gives only local infection. Serological detection of viola mottle virus in crude sap of V. odorata requires more sensitive methods than the slide precipitin test, which is not reliable with this host.
A virus with elongated particles from V. odorata in southern France, originally thought to be a potyvirus (Poupet & Marais, 1974), may be viola mottle virus (A. Poupet, personal communication).
Figures

Aggregates of virus-like particles in a systemically infected C. quinoa leaf cell. Bar represents 2 µm.
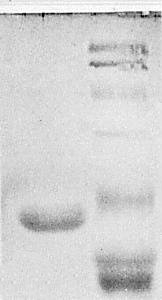
Electrophoresis in discontinuous SDS-polyacrylamide gels (Laemmli, 1970). Left lane: viola mottle virus protein. Right lane, from top to bottom (M. Wt in parentheses): bovine serum albumin (68,000), glutamate dehydrogenase (53,000), ovalbumin (43,000), carbonic anhydrase (29,000), a-chymotrypsinogen (25,700), tobacco mosaic virus coat protein (17,500) and cytochrome C (11,700).
References list for DPV: Viola mottle virus (247)
- Bancroft, Abou Haidar & Erickson, Virology 98: 121, 1979.
- Bancroft, Hills & Richardson, J. gen. Virol. 50: 451, 1980.
- Gould, Virology 108: 123, 1981.
- Laemmli, Nature, Lond. 227: 680, 1970.
- Lesemann & Koenig, in The Atlas of Insect and Plant Viruses, p. 331, ed. K. Maramorosch, New York: Academic Press, 478 pp., 1977.
- Lisa & Dellavalle, Phytopath. Z. 89: 82, 1977.
- Mowat, Rep. Scott. hort. Res. Inst. for 1978: 126, 1979.
- Phillips & Brunt, Acta Horticulturae 110: 65, 1980.
- Poupet & Marais, C.r. IVèmes Journées de Phytiatrie et de Phytopharmacie Circum-Mediterranéennes, Montpellier, 1974: 100, 1974.
- Richardson, Tollin & Bancroft, Virology 112: 34, 1981.
- Short, Rep. John Innes Inst. for 1980: 126, 1981.
- Tollin, Bancroft, Richardson, Payne & Beveridge, Virology 98:108, 1979.
- Tollin, Wilson & Bancroft, J. gen. Virol. 49: 407, 1980.
- Tollin, Wilson, Bancroft, Richardson, Payne & Alford, J. gen. Virol. 52: 205, 1981.