Details of DPV and References
DPV NO: 253 July 1982
Family: Potyviridae
Genus: Potyvirus
Species: Pepper mottle virus | Acronym: PepMoV
Pepper mottle virus
M. R. Nelson University of Arizona, Tucson, Arizona 85721, USA
R. E. Wheeler University of Arizona, Tucson, Arizona 85721, USA
T. A. Zitter Cornell University, Ithaca, New York 14853, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Nelson & Wheeler (1972), Zitter (1973), and Purcifull, Zitter & Hiebert (1975).
- A virus with flexuous, filamentous particles c. 737 nm long. It is transmitted by aphids in a non-persistent manner, is sap-transmissible, and causes mottle diseases of species of Capsicum and other solanaceous genera. Often occurs in pepper in mixed infections with other potyviruses.
Main Diseases
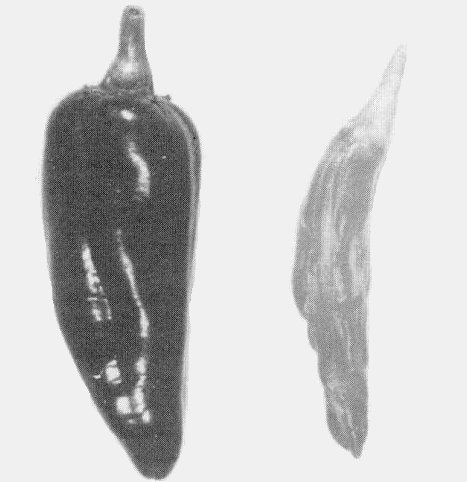
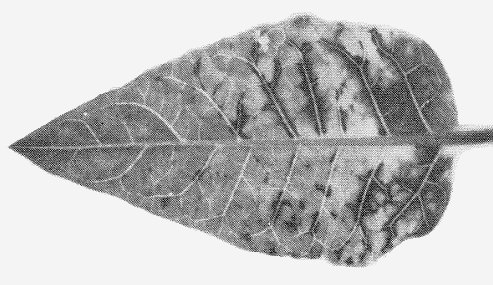
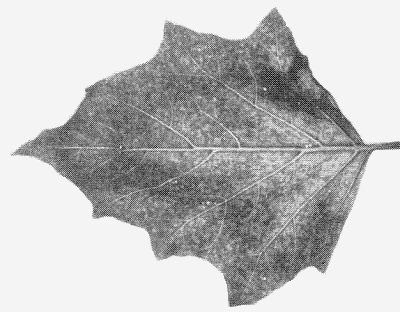
Causes mottle diseases of Capsicum annuum (sweet pepper; Fig. 1, Fig. 2) and C. frutescens (pungent pepper). Some strains cause considerable fruit distortion (Fig. 3, Fig. 4). Datura meteloides, the main overwintering host in Arizona (Nelson & Wheeler, 1978), shows mild mottle symptoms (Fig. 7).
Geographical Distribution
Common in certain areas of USA (Florida, New Mexico, Texas, Arizona and California) and in El Salvador.
Host Range and Symptomatology
Known to infect many species of Solanaceae, particularly of Capsicum and Nicotiana; some isolates produce local lesions in Chenopodium amaranticolor.
- Diagnostic species
- Capsicum annuum (sweet pepper) cvs Florida VR-2, Delray Bell and Agronomico-8. All develop
systemic mottle but Delray Bell expresses considerable tolerance. These cultivars are not infected
by common isolates of potato virus Y and tobacco etch virus. Cv. Early Calwonder shows severe systemic
mottle and pod symptoms.
- Capsicum frutescens cv. Tabasco. Most isolates induce necrotic lesions in inoculated leaves (Fig. 5) followed by systemic necrosis and death. A few isolates produce only a severe mottle. A Tabasco-type cultivar called Greenleaf Tabasco is not susceptible.
- Chenopodium amaranticolor. Some isolates induce chlorotic local lesions in inoculated leaves (Fig. 8), except when pepper extracts are used as inoculum. No systemic infection.
- Datura meteloides. Mild systemic mottle (Fig. 7).
- Nicotiana tabacum cv. V-20. Not susceptible to tested isolates.
- Capsicum frutescens cv. Tabasco. Most isolates induce necrotic lesions in inoculated leaves (Fig. 5) followed by systemic necrosis and death. A few isolates produce only a severe mottle. A Tabasco-type cultivar called Greenleaf Tabasco is not susceptible.
- Propagation species
- Most tobacco species and pepper varieties are useful for maintaining cultures and as a source
of virus for purification. Useful species of Nicotiana include N. tabacum cvs
Xanthi-nc (Fig. 6) and White Burley, as well as N. benthamiana, N. glutinosa and N.
glutinosa x N. clevelandii hybrid.
- Assay species
- Useful species include Chenopodium amaranticolor and Capsicum frutescens cv. Tabasco.
Strains
Commercial bell pepper and chilli types are susceptible to the type strain of the virus (Zitter & Ozaki, 1973) although tolerance has been reported (Zitter & Cook, 1973). Variants of the virus have been distinguished by the reactions of Capsicum annuum cv. Anaheim, C. frutescens cv. Tabasco, Chenopodium amaranticolor, Datura meteloides, Lycopersicon esculentum and Nicotiana tabacum cv. Xanthi-nc (Nelson & Wheeler, 1978; Zitter, 1973). One strain does not produce local lesions in pepper cv. Tabasco (Nelson & Wheeler, 1978).
Transmission by Vectors
Transmitted in a non-persistent manner by several species of aphid, including Myzus persicae. Both nymphs and adults of M. persicae can transmit the virus, and this species is probably the most efficient vector (Nelson & Wheeler, 1972; Zitter, 1975).
Transmission through Seed
None detected in the limited number of Capsicum cultivars tested (T. A. Zitter, M. R. Nelson & R. E. Wheeler, unpublished data).
Serology
The virus is a good immunogen. Crude extracts of infected plants treated with 1.5% sodium dodecyl sulphate (SDS) react with antiserum to virus particles in immunodiffusion tests in agar gels containing 0.5% SDS (Purcifull et al., 1975). They also react with antiserum specific for virus-induced lamellar inclusions (Purcifull, Hiebert & McDonald, 1973). The electron microscope antibody coating technique is also very useful (Nelson & Wheeler, 1979, 1981).
Relationships
The virus has been classified in the potyvirus group on the basis of its particle morphology, aphid transmissibility, ability to induce pinwheel inclusions in host cells, and distant serological relationship to potato virus Y (Nelson & Wheeler, 1972; Purcifull et al., 1975; Zitter, 1975). Immunodiffusion tests with SDS-treated extracts from infected plants indicate that pepper mottle virus is serologically distinct from, although related to, pepper veinal mottle virus, potato virus Y and tobacco etch virus. One of two pepper mottle virus antisera reacted weakly with pepper veinal mottle and potato Y viruses, but the homologous reaction produced an obvious spur (Purcifull et al., 1975). Pepper mottle virus antigens reacted weakly with one of three tobacco etch virus antisera, but failed to react with antisera to bidens mottle, lettuce mosaic, pepper veinal mottle and turnip mosaic viruses (Purcifull et al., 1975). In electron microscope antibody coating tests, there was no detectable serological relationship between pepper mottle, pepper veinal mottle, potato Y or tobacco etch viruses (M. R. Nelson & R. E. Wheeler, unpublished data).
Phenotypic mixing occurs naturally in mixed infections of pepper mottle and tobacco etch
viruses (Nelson & Wheeler, 1979, 1981): between 10 and 30% of particles from mixed infections
were only partly coated by antibody to either virus in immunoelectron microscopy tests (Fig. 11).
Stability in Sap
No information.
Purification
Turkish NN tobacco or Nicotiana hybrid (Christie, 1969) are suitable hosts (Purcifull et al., 1975) from which to purify both the virus particles and the virus-induced pinwheel inclusions from the same batch of tissue (Hiebert & McDonald, 1973). Homogenize infected tissue in 0.5 M potassium phosphate buffer, pH 7.5, containing 1% sodium sulphite (1 g tissue/1-2 ml buffer). To purify virus particles, clarify the homogenate by adding n-butanol to 8% (v/v) or chloroform to 10% (v/v). Centrifuge at low-speed, and precipitate the virus from the supernatant fluid by adding polyethylene glycol to 8% (v/v). Purify further by differential centrifugation and equilibrium centrifugation in CsCl. Inclusions were purified from leaf homogenates by a combination of treatments with Triton X-100 plus differential and sucrose density gradient centrifugation. Light microscopy is useful for monitoring the purification process and evaluating the purity of the extracts.
Properties of Particles
No information.
Particle Structure
Particles are flexuous filaments (Fig. 10), about 737 nm long (Nelson & Wheeler, 1972; Purcifull et al., 1975).
Particle Composition
Nucleic acid: No information.
Protein: Two electrophoretic components were observed in polyacrylamide/SDS gels, with estimated M. Wt of 28,000 and 34,000 (Hiebert & McDonald, 1973).
Relations with Cells and Tissues
The virus induces cytoplasmic pinwheel inclusions and scrolls which can be detected by light and electron microscopy (Fig. 9). The protein subunit of the pinwheel inclusion has a M. Wt of 67,000 (Hiebert & McDonald, 1973).
Notes
In America, pepper mottle virus frequently occurs in pepper in mixed infections with potato virus Y and tobacco etch virus. These three viruses can be distinguished by serology and by reactions in indicator plants. Although ordinary Tabasco pepper is susceptible to all three viruses (Nelson & Wheeler, 1978), Greenleaf Tabasco is resistant to pepper mottle and potato Y viruses, but is susceptible to at least some isolates of tobacco etch virus (Zitter, 1973). It can therefore be used to separate tobacco etch virus from a mixture with the other two viruses; this can also be done in Datura stramonium, which responds similarly. Potato virus Y can be freed from the other two viruses by passage through N. tabacum cv. V-20 (Christie, Purcifull & Dean, 1974); and pepper mottle virus can be separated from the mixture by passage through pepper cvs Florida VR-2, Delray Bell or Agronomico-8.
Pepper veinal mottle virus (Brunt & Kenten, 1972) occurs in pepper only in West Africa. It differs from tobacco etch, pepper mottle and potato Y viruses in not infecting Nicotiana tabacum cv. Xanthi-nc, and from pepper mottle virus in infecting Datura metel.
Figures

Electron micrograph of cytoplasmic inclusion bodies in ultra-thin section of tobacco leaf. The inclusions are seen longitudinally and in cross-section (the ‘pinwheel’). Note also an aggregate of virus particles (arrow). Bar represents 500 nm.
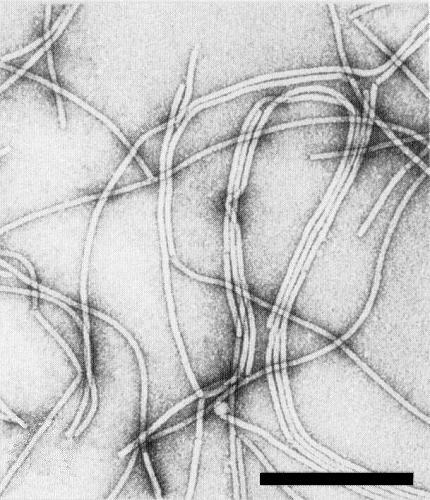
Purified virus particles in phosphotungstate, illustrating the extent of both lateral and end-to-end aggregation. Bar represents 300 nm.
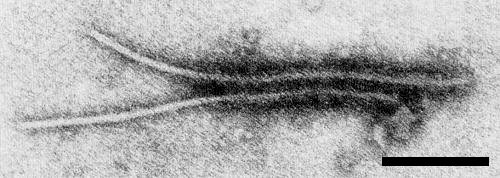
Phenotypic mixing, as revealed in the electron microscope by the antibody coating technique. A crude extract of Anaheim pepper infected with both pepper mottle virus and tobacco etch virus was treated with antiserum specific for pepper mottle virus. Only the right-hand portion of both particles is coated with antibody. Bar represents 200 nm.
References list for DPV: Pepper mottle virus (253)
- Brunt & Kenten, CMI/AAB Descr. Pl. Viruses 104, 4 pp., 1972.
- Christie, Pl. Dis. Reptr 53: 939, 1969.
- Christie, Purcifull & Dean, Pl. Dis. Reptr 58: 658, 1974.
- Hiebert & McDonald, Virology 56: 349, 1973.
- Nelson & Wheeler, Pl. Dis. Reptr 56: 731, 1972.
- Nelson & Wheeler, Phytopathology 68: 979, 1978.
- Nelson & Wheeler, Phytopathology 69: 918, 1979.
- Nelson & Wheeler, Phytopathology 71: 245, 1981.
- Purcifull, Hiebert & McDonald, Virology 55: 275, 1973.
- Purcifull, Zitter & Hiebert, Phytopathology 65: 559, 1975.
- Zitter, Pl. Dis. Reptr 57: 991, 1973.
- Zitter, Phytopathology 65: 110, 1975.
- Zitter & Cook, Phytopathology 63: 1211, 1973.
- Zitter & Ozaki, Proc. Fla St. hort. Soc. 86: 146, 1973.