Details of DPV and References
DPV NO: 255 July 1982
Family: Potyviridae
Genus: Potyvirus
Species: Peru tomato mosaic virus | Acronym: PTV
Peru tomato virus
C. E. Fribourg Dpto. de Fitopatologia, Universidad Nacional Agraria, Aptdo. 456, Lima, Peru
E. N. Fernandez-Northcote Dpto. de Fitopatologia, Universidad Nacional Agraria, Aptdo. 456, Lima, Peru
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
- Described by Raymer et al. (1972).
- A virus with flexuous elongated particles c. 750 x 12 nm. It is readily sap-transmissible and has a narrow host range. Transmitted in the non-persistent manner by Myzus persicae. Found in Peru.
Main Diseases
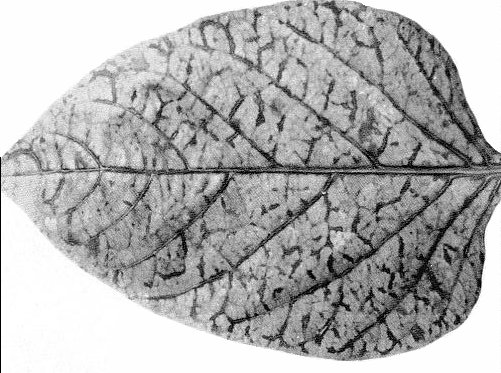
In tomato (Lycopersicon esculentum), the virus causes mosaic, epinasty, twisting, crinkling and necrotic spotting on the leaves (Fig. 1), and mosaic on the fruits. It also induces mosaic and veinbanding in sweet pepper (Capsicum annuum; Fig. 2), hot peppers (C. pendulum and C. chinense), and in Physalis peruviana.
Geographical Distribution
Reported only from Peru, where it is widely distributed along the irrigated coastal areas, inducing severe symptoms in tomato and pepper cultivars, and causing severe reductions in yield.
Host Range and Symptomatology
Natural infection has been detected in tomato, peppers, Physalis peruviana, Solanum nigrum and Nicandra physalodes. Except for a few chenopodiaceous species, which react with local lesions (Fig. 5), the host range is restricted to the family Solanaceae. No infection was obtained by sap inoculation of 84 species and 21 cultivars in 17 families (Fernandez-Northcote, 1978; Fernandez-Northcote & Fulton, 1980a; Fribourg, 1979).
- Diagnostic species
- Lycopersicon pimpinellifolium
. Leaf epinasty, stunting and systemic apical necrosis (Fig. 6). Some isolates induce mottle instead of necrosis. - Nicotiana debneyi. Systemic chlorotic rings and spots (Fig. 3).
- Nicotiana occidentalis. Irregular chlorotic blotches, mosaic and severe leaf deformation (Fig. 4).
- Solanum demissum x S. tuberosum A6, S. demissum Y, and potato (cvs Katahdin, Renacimiento, Mariva, Huancayo, Merpata, Participación) are not infected.
- Nicotiana occidentalis. Irregular chlorotic blotches, mosaic and severe leaf deformation (Fig. 4).
- Propagation species
- Nicotiana occidentalis
and N. tabacum (tobacco) cv. Burley 21, are suitable hosts for maintaining the virus and are good sources of virus for purification. Young N. occidentalis seedlings are suitable for aphid transmission tests.- Assay species
- Chenopodium amaranticolor
and C. quinoa are good local lesion hosts; however, a whole plant assay in Burley 21 tobacco is more sensitive.
Strains
Several strains that differ in virulence have been described. Fribourg (1979) found that isolate C induced systemic necrosis in L. pimpinellifolium whereas other isolates caused only stunting and mottle. Fernandez-Northcote (1978) and Fernandez-Northcote & Fulton (1980a) distinguished several further strains: strain M induces mosaic in Capsicum annuum cv. Avelar, whereas strain N induces systemic necrosis; strains OC, OM and OS failed to infect Avelar pepper, but differed in the severity of symptoms induced in tomato cv. Marglobe.
Transmission by Vectors
Transmitted by Myzus persicae after acquisition access periods of 30 sec and inoculation access times of 15 min.
Transmission through Seed
None detected. Seedlings derived from infected plants of tomato (several cultivars), pepper and Nicandra physalodes were virus-free.
Serology
The virus is moderately immunogenic; antiserum with a titre of 1/16,048 in microprecipitin tests was obtained after a prolonged course of intramuscular injections (Fernandez-Northcote & Fulton, 1980a). Flocculent precipitates are formed in microprecipitin tests and single lines in double diffusion tests in agar gels containing sodium dodecyl sulphate.
Relationships
The virus is classified in the potyvirus group on the basis of particle morphology, aphid-transmissibility, induction of cylindrical inclusions in infected cells, and serological relationship to some potyviruses. In microprecipitin and SDS-agar double diffusion tests, no serological differences were detected between strains of Peru tomato virus (SDI = 0) (Fernandez-Northcote & Fulton, 1980a). Using antiserum to Peru tomato virus in microprecipitin tests, Fribourg (1979) reported relationships to Colombian datura, henbane mosaic, pepper mottle, pepper veinal mottle, potato A, potato Y, tobacco etch and tobacco vein mottling viruses. Fernandez-Northcote & Fulton (1980a) reported SDI values of 5-8 between Peru tomato virus isolate M4 and pepper mottle, potato Y and tobacco etch viruses in microprecipitin tests; in addition, they detected a relationship to bidens mottle virus in SDS-agar double diffusion tests.
Stability in Sap
In Burley 21 tobacco sap the virus is inactivated by heating to 55-60°C for 10 min or by dilution beyond 10-5. It retained infectivity after storage for 10-21 days at 20-22°C. Infectivity survived for 3 years in tobacco leaves dried over anhydrous CaCl2.
Purification
The following method is effective (Fernandez-Northcote & Fulton, 1980a). Homogenize Burley 21 tobacco leaves in a mixture of chloroform and 0.5 M borate buffer, pH 7.6, containing 0.2% 2-mercaptoethanol (1 g leaf tissue: 1 ml chloroform: 2 ml buffer). Centrifuge at low speed and precipitate the virus from the supernatant fluid by adding polyethylene glycol M. Wt 6000 (to 5%) and NaCl (to 0.2 M). Centrifuge at low speed and disperse the pellet by shaking in 0.05 M borate and 0.01 M EDTA, pH 7.5. Concentrate the virus by two cycles of differential centrifugation. Further purify by rate-zonal centrifugation in sucrose gradients and resuspend in 0.05 M borate, pH 7.8. Yields vary from 14 to 50 mg virus per kg fresh leaves.
Properties of Particles
A single light-scattering zone is produced in sucrose density gradients. A260/A280: 1.28.
Particle Structure
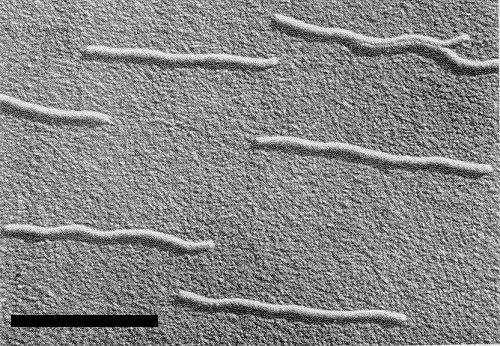
Particles are flexuous filaments with a modal length of c. 750 nm. Particle width is about 12 nm (Fig. 7).
Particle Composition
No reports.
Relations with Cells and Tissues
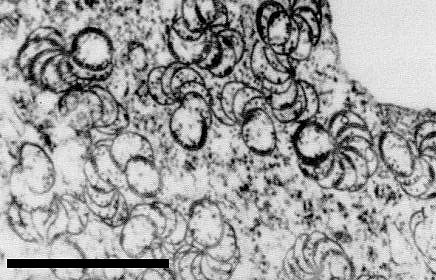
The virus induces characteristic cylindrical cytoplasmic inclusions observable by light microscopy in tissue stained with calcomine orange-luxol brilliant green (E. N. Fernandez-Northcote, unpublished data). In the electron microscope they appear in cross sections as pinwheels and scrolls (Fig. 8) and in longitudinal section as fibrous bundles (Fernandez-Northcote & Fulton, 1980b).
Notes
Peru tomato virus shares some properties with potato virus Y but it can be differentiated by serology and because it does not infect Solanum demissum x S. tuberosum A6, S. demissum Y or any of 6 potato cultivars tested,
Figures
References list for DPV: Peru tomato virus (255)
- Fernandez-Northcote, Ph.D. Thesis, Univ. Wisconsin, Madison, 1978.
- Fernandez-Northcote & Fulton, Phytopathology 70: 315, 1980a.
- Fernandez-Northcote & Fulton, Fitopatologia 15: 25, 1980b.
- Fribourg, Phytopathology 69: 441, 1979.
- Raymer, Kahn, Hikida & Waterworth, Phytopathology 62: 784, 1972.