Details of DPV and References
DPV NO: 156 September 1976
Family: Virgaviridae
Genus: Tobamovirus
Species: Tomato mosaic virus | Acronym: ToMV
Tomato mosaic virus
M. Hollings Glasshouse Crops Research Institute, Littlehampton, Sussex BN16 3PU, England
H. Huttinga Instituut voor Plantenziektenkundig Onderzoek, Binnenhaven 12, Postbus 42, Wageningen, The Netherlands
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by
Clinton (1909).
Synonyms
- Many synonyms have been used (see Martyn, 1968), including Lycopersicum virus 1 (Rev. appl. Mycol. 36: 303); see also under Strains
-
An RNA-containing virus, widespread and economically damaging in glasshouse and outdoor tomato crops in many countries. The virus is readily spread by handling and cultural operations; it also contaminates seeds and soil, but no natural vector is known. It is readily sap-transmissible to a fairly wide range of herbaceous plant species. The infective particles are straight rods c. 300 x 18 nm.
Main Diseases
Widespread and often epidemic in tomato (Lycopersicon esculentum) crops. Disease symptoms are greatly influenced by temperature, daylength, light intensity, age of plant, virus strain and cultivar of tomato (Ainsworth & Selman, 1936; Broadbent & Cooper, 1964; Crill et al., 1973; Fletcher & MacNeill, 1971; Paludan, 1968; Rast, 1967, 1975). Symptom types can be broadly grouped as:
1. Light and darker green mosaic leaf mottle, sometimes with distortion of younger leaves; this is the most common reaction in summer in glasshouses. In winter, with low light intensity, short days and temperatures not above 20°C, plants are often severely stunted and leaves distorted to ‘fern-leaf’ or tendril shape, but mottling may be slight. Plants are less vigorous, and fruit yield is reduced by 3 to 23% (Broadbent, 1964; Rast, 1975).
2. Conspicuous yellow or ‘aucuba’ mosaic leaf mottling (Bewley, 1923; Smith, 1957), which may also affect the fruit (Fig. 2).
3. Necrosis of stems, petioles, leaves and/or fruit. ‘Glasshouse streak’ (Jarrett, 1930) or ‘single virus streak’, consists of longitudinal necrotic streaks on stems or petioles, sometimes killing the plant; fruits develop large sunken necrotic lesions. Streak can occur at 26°C or below, with certain strains of the virus (Komuro et al., 1966), but can rarely be reproduced experimentally by sap-inoculation (Rast, 1975), although easily by grafting (van Dorst, 1963). ‘Double virus streak’ (Smith, 1957), caused by mixed infection with potato virus X plus almost any strain of tomato mosaic virus (Valleau & Johnson, 1930), is even more destructive than ‘single virus streak’, and the fruits are disfigured by irregular, raised necrotic lesions that later become sunken (Clinch, 1941). Internal necrosis or ‘bronzing’ in fruits results from infection with certain tomato mosaic virus strains when the fruits are developing (Boyle, 1971; Boyle & Bergman, 1967; Boyle & Wharton, 1957), and is distinguished from ‘blotchy ripening’ (Jenkins et al., 1965; Boyle, 1971) which may also be associated with tomato mosaic virus infections. Ribgrass mosaic virus can also induce fruit necrosis (Holmes, 1949). Other isolates of tomato mosaic virus cause winter necrosis, summer necrosis or crusty fruit diseases (Rast, 1975).
Sweet pepper (Capsicum frutescens) grown after affected tomato crops may show severe leaf necrosis and abscission, chronic mosaic and stunting (Fletcher, 1963). Tomato mosaic virus strains occur in Chenopodium murale in the USA, causing severe stunting, distortion and necrosis (Bald & Paulus, 1963), and in pear (Pyrus calleryana) associated with a diffuse chlorotic leaf spotting (Opel et al., 1969).
Geographical Distribution
World-wide, wherever tomatoes are grown.
Host Range and Symptomatology
Many solanaceous species are susceptible to the virus; most suscepts produce necrotic local lesions, sometimes followed by systemic mosaic-mottling and necrosis. Most species tested in the families Aizoaceae, Amaranthaceae, Chenopodiaceae and Scrophulariaceae are also susceptible (M. Hollings, unpublished data).
-
Diagnostic species
- Nicotiana glutinosa.
Dark, necrotic local lesions, initially 0.5 mm diameter, enlarging to c. 4 mm. Systemic invasion occurs at 33°C (M. Hollings, unpublished data), but different strains induce different symptoms: chlorosis and stunting; necrotic flecks and leaf distortion; or spreading necrosis. All strains are usually lethal within 1-2 weeks. - Several Nicotiana spp. have been used to distinguish tomato mosaic virus
strains from the type strain
or ‘tobacco’ forms of the virus,
though none is infallible.
- N. tabacum, White Burley type. Different selections respond very differently (Kassanis & Selman, 1947). In the necrotic or Dutch A selection (Termohlen & van Dorst, 1959; Broadbent, 1962), most typical tomato mosaic virus isolates induce necrotic local lesions in 3-4 days (Fig. 5) without systemic invasion, but some (including dahlemense, Ohio I, II and III strains) invade systemically (Fig. 6) (M. Hollings, unpublished data).
- N. sylvestris. Local lesions with no systemic invasion (Komuro et al., 1966; Wang & Knight, 1967; M. Hollings, unpublished data).
- N. rustica. Reported to react with necrotic local lesions only with all 18 tomato isolates tested (Rast, 1975); tobacco mosaic virus strains induce systemic mottle and necrosis. But 5 of 22 isolates (including dahlemense, Ohio I, II and III) caused systemic mottle and necrosis similar to tobacco isolates (M. Hollings, unpublished data).
- N. clevelandii x N. glutinosa hybrid (Christie, 1969). Gives only necrotic local lesions with eight of 22 tomato isolates (M. Hollings, unpublished data), and systemic lethal necrosis with 14 isolates.
- Datura stramonium. Necrotic local lesions after 2-3 days, with no systemic infection.
- Phaseolus vulgaris cv. Scotia. Some isolates (including dahlemense, Ohio I and II) induce reddish necrotic local lesions in 3-6 days in primary leaves, but others do not infect this plant (M. Hollings, unpublished data). Tobacco mosaic virus strains induce local lesions.
- Chenopodium spp. Chlorotic or necrotic local lesions (4-10 days) in C. amaranticolor, C. murale and C. quinoa (Fig. 3). Systemic pale green or necrotic flecks and mottle in C. murale; most isolates behave likewise in C. amaranticolor, and some do in C. quinoa. Systemic symptoms ranged from severe to none, and were not distinguishable from those induced by tobacco mosaic virus (M. Hollings, unpublished data).
- Gomphrena globosa. Necrotic or semi-necrotic local lesions in c. 1 week; most isolates induce systemic chlorotic mottle.
- Tetragonia expansa. Chlorotic local lesions in 7-10 days, subsequently enlarging to characteristic necrotic-edged lesions. Some isolates invade systemically, with or without symptoms.
- Some tomato isolates cause symptomless infection in inoculated leaves of Brassica pekinensis or cotyledons of Cucumis sativus cv. Butcher’s Disease Resister. No infection in Phaseolus vulgaris cv. The Prince or Vigna sinensis cv. Blackeye.
- Tobacco mosaic virus, in contrast to tomato mosaic virus, typically induces systemic disease in N. sylvestris, N. tabacum White Burley necrotic selection, and D. stramonium.
Propagation species
- Nicotiana clevelandii.
Small plants are rapidly killed and yield little virus; large plants develop necrotic local lesions followed by systemic rosetting and crinkling, with severe general chlorosis. High yields of virus are obtained, but plants are usually killed in 2-4 weeks. Tomato is unsuitable for propagating or maintaining the virus because it gives low yields, preparations contain more impurity, and it can selectively change the character of isolates (see below). Cultures are best maintained at -20°C in leaves or sap. - N. tabacum, White Burley type. Different selections respond very differently (Kassanis & Selman, 1947). In the necrotic or Dutch A selection (Termohlen & van Dorst, 1959; Broadbent, 1962), most typical tomato mosaic virus isolates induce necrotic local lesions in 3-4 days (Fig. 5) without systemic invasion, but some (including dahlemense, Ohio I, II and III strains) invade systemically (Fig. 6) (M. Hollings, unpublished data).
-
Assay species
- Nicotiana glutinosa
is widely used; N. tabacum cvs. Xanthi-nc or Samsun NN, Chenopodium quinoa and C. murale are also suitable.
Strains
Many isolates of tomato mosaic virus are similar in amino acid composition, base composition and buoyant density, and are serologically closely related (Wang & Knight, 1967; Mosch et al., 1973; Dawson et al., 1975; Hollings, 1974 and unpublished data), although they may differ in symptomatology. A few isolates differ from the majority in amino acid composition or serological behaviour.
One way in which strains have been classified is by their ability to induce symptoms in plants of certain Lycopersicon spp., or in isogenic lines of Craigella tomato (Fig. 1), possessing resistance genes Tm-1, Tm-2 or Tm-22 (Clayberg et al., 1960; Pelham, 1972; Dawson et al., 1975). Five ‘Pelham groups’ were defined by ability to overcome the genes specified: 0 (unable to overcome any resistance genes), 1 (gene Tm-1), 2 (gene Tm-2), 1.2 (genes Tm-I and Tm-2), 22 (gene Tm-22). Thus, of the Ohio strains (McRitchie & Alexander, 1963; Cirulli & Alexander, 1969; Alexander, 1971), I and II are in Pelham group 0, III is in group 1, IV in group 2, and V in group 1.2.
‘Common tomato mosaic’ isolates include representatives in all Pelham’s categories. Most tomato isolates are of Pelham type 0; strain 22, able to overcome resistance gene Tm-22, has only recently been detected (Rast, 1975). Field isolates may contain virus types in more than one group (Alexander, 1971; Rast, 1975), and a strain may change markedly on passage through some tomato genotypes (Zitter & Murakishi, 1969; Pelham et al., 1970; MacNeill & Fletcher, 1971; MacNeill & Boxall, 1974).
These categories are of more value to plant breeders than to virologists; they do not coincide with groupings based on antigenic relatedness, differential reactions in Nicotiana species, or symptoms in tomato. Nor do they fully equate with the system of Rast (1975), who placed 115 virus isolates in four groups according to their reactions in Solanum penelli and clonal lines of certain Lycopersicon spp. Thus, 14 isolates of Pelham type 0 were serologically quite closely related to one another, as well as to most isolates in Pelham groups 1 (Pelham type strain 1, dahlemense and M II-16 strains), 2 and 1.2 (M. Hollings, unpublished data). In contrast, strains Ohio I and II are very similar to tobacco mosaic virus in amino acid composition (Dawson et al., 1975), reactions in Nicotiana spp. and serological behaviour (M. Hollings, unpublished data), but they differ from it in being well adapted to tomato. Strain Ohio III differed both from tobacco mosaic virus and from tomato mosaic virus in protein composition (Dawson et al., 1975), and serological behaviour (Fig. 4) (M. Hollings, unpublished data). Ohio strain IV, however, is very closely related to Pelham type strain 2 (M. Hollings, unpublished data). Using cross-protection tests, Oshima (1973) grouped isolates Chiba 2, 3 and 4 with strain Ohio IV, and separated these from Ohio I, II, III and V. Strains Ohio I, II and IV (Alexander, 1962) and Ohio III (Rast, 1968) have been identified in Europe.
Strains have also been named from symptoms in tomato; these strains include:
Tomato aucuba mosaic strain (Bewley, 1923; Henderson Smith, 1928); can be Pelham type 0, 1 or 2 (M. Hollings & B. J. Thomas, unpublished data; B. H. MacNeill, personal communication). Causes a conspicuous yellow or ‘aucuba’ leaf mottle, and sometimes mottled fruits (Fig. 2).
Tomato enation mosaic strain (Ainsworth, 1937); causes leaf distortion and enations.
Dahlemense strain (Melchers, 1940); tends to produce yellow mosaic mottling, is in Pelham group 1, and reacts in Nicotiana test plants more like tobacco mosaic virus (M. Hollings, unpublished data).
Yellow ringspot strain (Rast, 1965; 1975); induces yellow ringspots, and is of Pelham type 0.
Winter necrosis strain (Rast, 1975); Pelham type 2.
Summer necrosis strain (Rast, 1975); Pelham type 1. Possibly equates with tomato streak strain of Komuro (1963).
Tomato crusty fruit strain (Rast, 1975); causes corky crusts on fruit; induces very tiny local lesions in Nicotiana glutinosa, and is of Pelham type 0.
Tomato rosette strain (Price & Fenne, 1951); severe distortion and stunting similar to hormone weedkiller damage.
Tomato black fleck strain (Hollings, 1957); induces black necrotic leaf flecks. Pelham type 0.
M II-16 strain (Rast, 1972); a nitrous acid mutant of low pathogenicity, widely used for protective inoculation of tomato seedlings; Pelham type 1.
Transmission by Vectors
No natural specific vector known, although grasshoppers transmitted experimentally; early reports of aphid transmission are now regarded as due to inoculation by means of aphids’ claws (Bradley & Harris, 1972). Man is the principal vector. Infection is most often introduced into tomato or pepper crops through root infection from contaminated soil (Doolittle, 1928; Broadbent, 1965a; Fletcher, 1969) and/or through contaminated seedlings. From these infection foci, the virus is readily spread by leaf contact and cultural operations (Broadbent, 1965a, 1965b; Broadbent & Fletcher, 1966).
Transmission through Seed
The virus is present in the external mucilage, testa and sometimes endosperm of tomato seeds, but was not proved to be within the embryo (Taylor et al., 1961; Broadbent, 1965b). The percentage of contaminated seeds varies greatly in different fruits; up to 94% of seeds may contain the virus (van Winckel, 1965). Infection of seedlings occurs during transplanting, but not if seedlings are undisturbed (Broadbent, 1965b).
The virus is readily eliminated from the outside of tomato seeds by soaking them for 20 min in 10% (w/v) Na3PO4 solution; all external and usually all internal virus is eliminated by heat treatment of dry seeds (2-4 days at 70°C) without impairing seed germination (Howles, 1961; Broadbent, 1965b). Some samples of infected seed lost all virus when stored 7 months in paper packets, but not when stored in bulk (John & Soya, 1955); seed samples with endosperm infection remained so for at least 9 years (Broadbent, 1965b).
Transmission by Dodder
‘Yellow’ and ‘green’ strains of the virus were transmitted in winter, but rarely in summer, by Cuscuta subinclusa and two other Cuscuta spp. (Schmelzer, 1956).
Serology
The virus is a fairly good immunogen; one intravenous, followed by two intramuscular injections (with complete adjuvant), each of c. 3 mg virus, gave antisera with precipitin titres of 1/4000 to 1/16,000 (M. Hollings, unpublished data). The virus reacts satisfactorily in precipitin (tube or micro), ring, and gel-diffusion (freshly prepared 0.8% Ionagar No. 2) tests. One major precipitation line in gel is often accompanied by a second, weaker line (Fig. 4); multiple precipitation lines are discussed by Kleczkowski (1968).
Relationships
Tomato mosaic virus is a characteristic member of the tobamovirus group. Typical strains are more closely related to type strain tobacco mosaic virus than to other tobamoviruses, but can be differentiated from them all by differential host responses, serological reactions and amino acid composition of the virus protein. The extent of serological relatedness among several tobamoviruses was correlated with amino acid sequence homology (van Regenmortel, 1975).
In tomato, mild strains of tomato mosaic virus usually protect against more severe strains (Boyle & Bergman, 1969; Rast, 1972); tobacco mosaic virus, however, does not protect against tomato mosaic virus in tomato (Broadbent & Winsor, 1964), nor does tomato mosaic virus protect against tobacco mosaic virus in tobacco (Jensen, 1969). Ribgrass mosaic virus does not protect against tomato mosaic virus in tomato (Broadbent, 1964).
Stability in Sap
In tomato sap, the virus loses infectivity after 10 min at 85-90°C, or after dilution to 10-5 to 10-6. Nicotiana clevelandii sap may still be infective at 2 x 10-7. In air-dried tomato leaf, the virus was still infective after 24 years at laboratory temperature (Caldwell, 1959). Sap retains infectivity for many months at laboratory temperature and for several years at 0 to -2°C, although some isolates show marked changes in virulence after such storage (Rast, 1975). Storage at -20°C, or after lyophilisation, seems to prevent this.
Purification
Readily purified in large amounts (up to 160 mg virus/kg tissue being obtained with most isolates; M. Hollings, unpublished data). A good method that requires no ultracentrifuge is a modification of Gooding & Hebert’s procedure (1967) : harvest Nicotiana clevelandii plants 10-14 days after infection, freeze overnight, and homogenise in 0.05 M phosphate buffer (pH 7.4) containing 0.1% (v/v) thioglycollic acid (4 ml buffer/g tissue). Squeeze out sap through cloth, add n-butanol dropwise (9.3 ml/100 ml juice) and shake for 45 min at laboratory temperature. Clarify (30 min centrifugation, 10,000 g) and to the supernatant fluid add 4 g polyethylene glycol (PEG, M. Wt 6000) per 100 ml supernatant fluid; stir until dissolved, and stand mixture c. 30 min, then sediment virus precipitate (15 min, 10,000 g), and re-suspend pellet in 0.01 M phosphate buffer (20 ml/100 ml of initial extractant). Clarify (15 min, 10,000 g). Further purification can be obtained by a second precipitation with PEG (add 4.0 g NaCl, then 4.0 g PEG/100 ml fluid and stir until dissolved); sediment virus precipitate (15 min, 10,000 g) and re-suspend pellet in 2 ml 0.01 M phosphate buffer/100 ml initial extract; remove insoluble material by brief centrifugation (5 min, 10,000 g).
Considerably purer preparations can be made by application of 1.0 to 1.5 ml portions of the above preparations to columns (85 x 1.5 cm) of controlled pore glass beads (70 nm pore size; Sigma London Chemical Co. Ltd.), pretreated with PEG M. Wt 20,000 at 1% (w/v) in 0.04 M phosphate buffer pH 7.0. The virus is eluted with 0.04 M phosphate buffer (pH 7.0) and appears in the void volume, well separated from host material (M. Hollings & R. J. Barton, unpublished data). The virus can be further concentrated by precipitation with ethanol (2.5:1 v/v) and centrifuging the virus precipitate.
Properties of Particles
Purified preparations sediment as a major infective component, sometimes also with dimers and trimers.
Sedimentation coefficient of monomers (s°20, w): 177 S (Mosch et al., 1973); 190 S (M. Hollings & A. A. Brunt, unpublished data).
Isoelectric point: pH 4.64 for the yellow aucuba strain, and pH 4.50 for a green mutant from this strain (Oster, 1951).
A260/A280: 1.17. Amax(261)/Amin(249): 1.10 (M. Hollings, unpublished data); both values corrected for light-scattering.
Buoyant density: slightly higher for 18 strains of tomato mosaic virus than for tobacco mosaic virus, type strain (Mosch et al., 1973).
Electrophoretic mobility: -0.97 (µm/sec) (V/cm) at pH 7.0 and 0.075 ionic strength (dahlemense strain) (Kramer & Wittmann, 1958).
Particle Structure
In 2% phosphotungstate, infective particles are straight tubules c. 300 x 18 nm, and having a periodically deformed helical structure (Caspar & Holmes, 1969).
Particle Composition
Nucleic acid: RNA, single-stranded, about 5% of particle weight, probably c. 2 x 106 M. Wt. Nucleotide base ratios of 18 isolates of the virus averaged: G 23: A28: C 19: U 30 moles % (Mosch et al., 1973).
Protein: In polyacrylamide gel electrophoresis, four strains of tomato mosaic virus contained one polypeptide of estimated M. Wt (hydrated) c. 21,000 (M. Hollings & R. J. Barton, unpublished data). Similar amino acid compositions were determined for 36 strains (Wang & Knight, 1967; Mosch et al., 1973; Dawson et al., 1975). The amino acid sequence of the dahlemense strain (Wittmann-Liebold & Wittmann, 1967) contained 158 residues with a M. Wt of 17,640:
10
AcSer -Tyr-Ser-Ile-Thr-Ser-Pro-Ser-Gln-
Phe-Val-Phe-Leu-Ser-Ser-Val-Trp-Ala-Asp-
20 30
Pro-Ile-Glu-Leu-Leu-Asn-Val-Cys-Thr-Ser-
Ser-Leu-Gly-Asn-Gln-Phe-Gln-Thr-Gln-Gln-
40 50
Ala-Arg-Thr-Thr-Val-Gln-Gln-Gln-Phe-Ser-
Glu-Val-Trp-Lys-Pro-Phe-Pro-Gln-Ser-Thr-
60 70
Val-Arg-Phe-Pro-Gly-Asp-Val-Tyr-Lys-Val-
Tyr-Arg-Tyr-Asn-Ala-Val-Leu-Asp-Pro-Leu-
80 90
Ile-Thr-Ala-Leu-Leu-Gly-Thr-Phe-Asp-Thr-
Arg-Asn-Arg-Ile-Ile-Glu-Val-Glu-Asn-Gln-
100 110
Gln-Ser-Pro-Thr-Thr-Ala-Glu-Thr-Leu-Asp-
Ala-Thr-Arg-Arg-Val-Asp-Asp-Ala-Thr-Val-
120 130
Ala-Ile-Arg-Ser-Ala-Ile-Asn-Asn-Leu-Val-
Asn-Glu-Leu-Val-Arg-Gly-Thr-Gly-Leu-Tyr-
140 150
Asn-Gln-Asn-Thr-Phe-Glu-Ser-Met-Ser-Gly-
Leu-Val-Trp-Thr-Ser-Ala-Pro-Ala-Ser
There are 30 amino acid exchanges from the type strain of tobacco mosaic virus. The methionine is in a different position from that in ribgrass mosaic virus.
Relations with Cells and Tissues
The virus occurs in all tissues, including pollen and seeds, although probably not in embryos (Taylor et al., 1961; Broadbent, 1965b). Intracellular inclusions, recorded with different strains and in many natural and experimental hosts, include: crystalline inclusions, amorphous bodies that become vacuolate (Henderson Smith, 1930), hexagonal crystals, fine needles, fibrous spikes, spindle bodies, long curved fibres, and amoeboid or X-bodies (Kassanis & Sheffield, 1941; Allen, 1969); also ‘angled layer’ aggregates (Warmke, 1968). Aucuba mosaic strain has been studied most, and amoeboid bodies formed with this strain (Sheffield, 1931) are now thought to differ from the X-bodies induced by tobacco mosaic virus, type strain (Warmke, 1969).
Notes
Most isolates from tomato are of tomato mosaic virus type; tobacco mosaic virus strains are seldom found (Broadbent, 1962; MacNeill, 1962; Komuro et al., 1966), for most of them compete poorly in tomato (Komuro et al., 1966; Jensen, 1969; Tomaru et al., 1970), and some (e.g. U2) infect tomato with difficulty or not at all (Bald & Paulus, 1963).
Tomato mosaic virus can be distinguished from other viruses affecting tomato as follows. Tomato mosaic virus local lesions in Nicotiana glutinosa differ in size and incubation period from those of tomato spotted wilt and tomato bushy stunt viruses. Cucumber mosaic and tomato aspermy viruses cause systemic mottle and distortion in N. glutinosa and necrotic local lesions in Vigna sinensis. Tomato black ring virus induces systemic mottle in Phaseolus vulgaris and Cucumis sativus; tomato ringspot virus gives symptoms similar to those of tomato black ring virus in C. sativus but leaf-tip necrosis and rugose mottle in P. vulgaris. Potato virus Y incites systemic vein clearing mottle in N. glutinosa; potato virus X produces systemic chlorotic rings and mottle in Datura stramonium.
Attempts to control tomato mosaic disease have been based on: (1) production of virus-free (heat-treated) tomato seed; (2) milk or other sprays applied to foliage to inhibit mechanical transmission of the virus (Crowley, 1958; Hare & Lucas, 1959; Denby & Wilks, 1963; Hein, 1964; Jaeger, 1966). (3) Soil sterilization, which is usually inadequate to prevent subsequent root infection (Broadbent, 1965a; Broadbent & Winsor, 1964; Broadbent et al., 1965; van den Brock et al., 1967; Fletcher, 1969), although all root infections do not necessarily progress to the shoots (Komuro & Iwaki, 1969; Rast, 1973). (4) Inoculation of tomato seedlings with selected or induced mild strains of the virus (Goto et al., 1966; Paludan, 1968, 1973; Boyle & Bergman, 1969; Marrou & Migliori, 1971; Rast, 1972; Fletcher & Rowe, 1975). Mild strains may recover their virulence (Oshima & Goto, 1968), however, or may change the relative prevalence of strain types in subsequent tomato crops (Fletcher & Butler, 1975). (5) The use of genetically resistant tomato cvs (Pelham, 1966; Alexander & Oakes, 1970; Alexander & Farley, 1972; Gates & McKeen, 1972); single-gene resistance was soon overcome (Dawson, 1967; Pelham et al., 1970), but the three-gene resistant cvs ‘Kirdford Cross’ and ‘Pagham Cross’ are still effective (Thomas, 1973).
Figures

Tomato mosaic virus (dahlemense strain) in Craigella tomato seedling, 18 days after infection (left), showing local lesions in cotyledons, systemic chlorotic mottle and severe stunting. Healthy plant (right). This also illustrates the method of typing virus isolates by reactions in various isogenic lines of cv. Craigella containing different combinations of resistance genes. This isolate has infected and stunted plants containing gene Tm-1, and is thus in Pelham group 1 (Pelham, 1972).

Symptoms of the aucuba mosaic strain in fruit of Moneymaker tomato, visible as a light and dark green blotch-mottle.
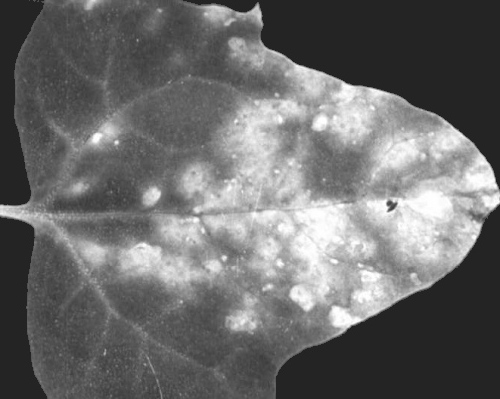
Yellowish local lesions of a typical tomato mosaic virus isolate in Chenopodium quinoa leaf 12 days after inoculation.

Relationships among some strains of the virus in immunodiffusion tests. Central well contains antiserum to typical tomato mosaic strain AC; antigen wells contain purified preparations of: O=Ohio III; T2 = Pelham strain T2; M = typical tomato strain of Pelham type 0; T1.2 = Pelham strain T1.2; D = dahlemense strain (a Pelham type 1 strain). Dahlemense and T1.2 are closely related, whereas T2 and M share only some antigenic determinants; Ohio III is distantly related to the other four strains.
References list for DPV: Tomato mosaic virus (156)
- Ainsworth, Ann. appl. Biol. 24: 545, 1937.
- Ainsworth & Selman, Ann. appl. Biol. 23: 89, 1936.
- Alexander, Meded. LandbHoogesch. Opzoek Stns Gent 27: 1020, 1962.
- Alexander, Phytopathology 61: 611, 1971.
- Alexander & Farley, Res. Bull. Ohio Res. Dev. Cent. 1057: 3, 1972.
- Alexander & Oakes, Phytopathology 60: 1281, 1970.
- Allen, Diss. Abstr. 29: B-3633, 1969.
- Bald & Paulus, Phytopathology 53: 627, 1963.
- Bewley, Diseases of Glasshouse Plants: 195 pp., Ernest Benn, London, 1923.
- Boyle, Phytopathology 61: 127, 1971.
- Boyle & Bergman, Phytopathology 57: 354, 1967.
- Boyle & Bergman, Phytopathology 59: 397, 1969.
- Boyle & Wharton, Phytopathology 47: 199, 1957.
- Bradley & Harris, Virology 50: 615, 1972.
- Broadbent, Ann. appl. Biol. 50: 461, 1962.
- Broadbent, Ann. appl. Biol. 54: 209, 1964.
- Broadbent, Ann. appl. Biol. 55: 57, 1965a.
- Broadbent, Ann. appl. Biol. 56: 177, 1965b.
- Broadbent & Cooper, Ann. appl. Biol. 54: 31, 1964.
- Broadbent & Fletcher, Ann. appl. Biol. 57: 113, 1966.
- Broadbent, Read & Last, Ann. appl. Biol. 55: 471, 1965.
- Broadbent & Winsor, Ann. appl. Biol. 54: 23, 1964.
- Caldwell, Nature, Lond. 183: 1142, 1959.
- Caspar & Holmes, J. molec. Biol. 46: 99, 1969.
- Christie, Pl. Dis. Reptr 53: 939, 1969.
- Cirulli & Alexander, Phytopathology 59: 1287, 1969.
- Clayberg, Butler, Rick & Young, J. Hered. 51: 167, 1960.
- Clinch, J. Dept. Agric. Repub. Ire. 38: 3, 1941.
- Clinton, Rep. Conn. agric. Exp. Stn 1907-1908: 854, 1909.
- Crill, Burgis, Jones & Strobel, Pl. Dis. Reptr 57: 78, 1973.
- Crowley, J. Austr. Inst. agric. Sci. 24: 261, 1958.
- Dawson, Ann. appl. Biol. 60: 209, 1967.
- Dawson, Rees & Short, Ann. appl. Biol. 79: 189, 1975.
- Denby & Wilks, Can. J. Pl. Sci. 43: 457, 1963.
- Doolittle, Phytopathology 18: 155, 1928.
- Fletcher, Pl. Path. 12: 113, 1963.
- Fletcher, Pl. Path. 18: 97, 1969.
- Fletcher & Butler, Ann. appl. Biol. 81: 409, 1975.
- Fletcher & MacNeill, Can. J. Pl. Sci. 51: 101, 1971.
- Fletcher & Rowe, Ann. appl. Biol. 81: 171, 1975.
- Gates & McKeen, Can. Pl. Dis. Surv. 52: 33, 1972.
- Gooding & Hebert, Phytopathology 57: 1285, 1967.
- Goto, Komochi & Oshima, Ann. phytopath. Soc. Japan 32: 221, 1966.
- Hare & Lucas, Pl. Dis. Reptr 43: 152, 1959.
- Hein, Z. PflKrankh. PflPath. PflSchutz 71: 206, 1964.
- Henderson Smith, Ann. appl. Biol. 15: 155, 1928.
- Henderson Smith, Ann. appl. Biol. 17: 213, 1930.
- Hollings, Pl. Path. 6: 133, 1957.
- Hollings, Acta Hort. 36: 23, 1974.
- Holmes, Pl. Dis. Reptr 33: 338, 1949.
- Howles, Pl. Path. 10: 160, 1961.
- Jaeger, Phytopath. Z. 56: 340, 1966.
- Jarrett, Ann. appl. Biol. 17: 248, 1930.
- Jenkins, Wiggell & Fletcher, Ann. appl. Biol. 55: 71, 1965.
- Jensen, Diss. Abstr. 29: B-3991, 1969.
- John & Sova, Phytopathology 45: 636, 1955.
- Kassanis & Selman, J. Pomol. 23: 167, 1947.
- Kassanis & Sheffield, Ann. appl. Biol. 28: 360, 1941.
- Kleczkowski, Virology 36: 700, 1968.
- Komuro, Ann. phytopath. Soc. Japan 28: 40, 1963.
- Komuro & Iwaki, Ann. phytopath. Soc. Japan 35: 294, 1969.
- Komuro, Iwaki & Nakahara, Ann. phytopath. Soc. Japan 32: 130, 1966.
- Kramer & Wittmann, Z. Naturforsch. 13b: 30, 1958.
- MacNeill, Can. J. Bot. 40: 49, 1962.
- MacNeill & Boxall, Can. J. Bot. 52: 1305, 1974.
- MacNeill & Fletcher, Can. J. Bot. 49: 1947, 1971.
- McRitchie & Alexander, Phytopathology 53: 394, 1963.
- Marrou & Migliori, Annls Phytopath. 3: 447, 1971.
- Martyn, Plant Virus Names, CMI, Kew, 1968.
- Melchers, Biol. Zbl. 60: 527, 1940.
- Mosch, Huttinga & Rast, Neth. J. Pl. Path. 79: 104, 1973.
- Opel, Kegler & Richter, Acta phytopath. Acad. Sci. hung. 4: 1, 1969.
- Oshima, Rep. Tottori mycol. Inst. 10: 771, 1973.
- Oshima & Goto, Ann. phytopath. Soc. Japan 34: 263, 1968.
- Oster, J. biol. Chem. 190: 55, 1951.
- Paludan, Tidsskr. PlAvl. 72: 69, 1968.
- Paludan, Tidsskr. PlAvl. 77: 495, 1973.
- Pelham, Euphytica 15: 258, 1966.
- Pelham, Ann. appl. Biol. 71: 219, 1972.
- Pelham, Fletcher & Hawkins, Ann. appl. Biol. 65: 293, 1970.
- Price & Fenne, Phytopathology 41: 1091, 1951.
- Rast, Neth. J. Pl. Path. 71: 91, 1965.
- Rast, Neth. J. Pl. Path. 73: 147, 1967.
- Rast, Neth. J. Pl. Path. 74: 234, 1968.
- Rast, Neth. J. Pl. Path. 78: 110, 1972.
- Rast, Neth. J. Pl. Path. 79: 5, 1973.
- Rast, Meded. Inst. plziektenk. Onderz. 689, 76 pp., 1975.
- Sheffield, Ann. appl. Biol. 18: 471, 1931.
- Schmelzer, Phytopath. Z. 28: 1, 1956.
- Smith, Textbook of Plant Virus Diseases, 2nd Ed., Churchill, 1957.
- Taylor, Grogan & Kimble, Phytopathology 51: 837, 1961.
- Termohlen & van Dorst, Jversl. Proefstn Groente- en Fruitteelt Glas Naaldwijk 1958: 125, 1959.
- Thomas, Rep. Glasshouse Crops Res. Inst. 1972: 30, 1973.
- Tomaru, Suyama & Kubo, Ann. phytopath. Soc. Japan 36: 74, 1970.
- Valleau & Johnson, Phytopathology 20: 831, 1930.
- van den Brock, Vanachter & van Winckel, Agricultura, Louvain 15: 120, 1967.
- van Dorst, Jversl. Proefstn Groente- en Fruitteelt Glas Naaldwijk 1962: 143, 1963.
- van Regenmortel, Virology 64: 415, 1975.
- van Winckel, Agricultura, Louvain 13: 721, 1965.
- Wang & Knight, Virology 31: 101, 1967.
- Warmke, Virology 34: 149, 1968.
- Warmke, Virology 39: 695, 1969.
- Wittmann-Liebold & Wittmann, Mol. gen. Genetics 100: 358, 1967.
- Zitter & Murakishi, Phytopathology 59: 1736, 1969.

