Details of DPV and References
DPV NO: 267 July 1983
Family: Secoviridae
Genus: Nepovirus
Species: Blueberry leaf mottle virus | Acronym: BLMoV
Blueberry leaf mottle virus
D. C. Ramsdell Department of Botany & Plant Pathology, Michigan State University, East Lansing, Michigan, 48824-1312 USA
R. Stace-Smith Agriculture Canada Research Station, Vancouver, British Columbia, Canada V6T 1X2
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
Main Diseases
Causes a severe dieback of the main stems of highbush blueberry (Vaccinium corymbosum) cvs. Rubel and Jersey, with leaf mottling (Fig. 1) and sometimes leaf deformation (Fig. 2). Symptoms are the most pronounced on cv. Rubel (Ramsdell & Stace-Smith, 1979). In Vitis labrusca cv. Concord, the virus causes delayed bud break, irregular elongation of fruits, pale green foliage and straggly fruit clusters (Uyemoto et al., 1977; see also Description No. 186).
Geographical Distribution
Michigan and New York States, USA.
Host Range and Symptomatology
Transmitted experimentally by inoculation of sap to a narrow range of hosts comprising 23 species in seven dicotyledonous families (Ramsdell & Stace-Smith, 1979; Uyemoto et al. , 1977); symptomless infection occurred in nine of these species (Ramsdell & Stace-Smith, 1979). When purified virus preparations were used as inoculum, the MI (blueberry) strain infected seedlings of cv. Rubel highbush blueberry (Fig. 3) but the NY (grapevine) strain did not (Ramsdell & Stace-Smith, 1979). A low percentage of seedlings of American grape (Vitis labrusca) cv. Niagara were infected by inoculation with the MI strain (Ramsdell & Stace-Smith, 1980).
- Diagnostic species
- Chenopodium quinoa. In inoculated leaves,
chlorotic local lesions are formed within 7-10 days
(Fig. 4). Symptoms consist of mottling and epinasty of
terminal leaves, followed by death of the apex.
- Nicotiana clevelandii. Necrotic ringspots form in inoculated leaves. Younger leaves develop localized systemic necrotic spotting (Fig. 5). Symptoms occur in 14-21 days.
- Nicotiana clevelandii. Necrotic ringspots form in inoculated leaves. Younger leaves develop localized systemic necrotic spotting (Fig. 5). Symptoms occur in 14-21 days.
- Propagation and assay species
- Both C. quinoa and N. clevelandii are good propagation hosts. Only C. quinoa is satisfactory as a local lesion host
Strains
Two strains have been distinguished. The MI strain, found in blueberry in Michigan (Ramsdell & Stace-Smith, 1979), has slight serological differences from the NY strain, found in grapevine in New York State (Uyemoto et al., 1977). Although the two strains may differ in ability to infect blueberry and grapevine (see Host Range and Symptomatology) they cannot be distinguished on herbaceous hosts.
Transmission by Vectors
Although the virus resembles nepoviruses in many properties, no success was obtained in attempts to transmit the MI strain by hand-picked Xiphinema americanum to C. quinoa or N. clevelandii test plants, whether the nematodes were from a Michigan source (T. Vrain & D. C. Ramsdell, unpublished data) or from an Arkansas source (J. McGuire & D. C. Ramsdell, unpublished data). Under the same conditions, the Arkansas nematodes transmitted tobacco ringspot virus. Uyemoto et al. (1977) found no transmission of the NY strain to bait seedlings of C. quinoa or cucumber planted in X. americanum-infested soil taken from beneath a diseased Concord grapevine.
Transmission through Seed
The NY strain is transmitted through seeds to seedlings of Vitis labrusca (c. 5%) and C. quinoa (12%) (Uyemoto et al. , 1977). The MI strain is seed-borne in 20% of C. quinoa seeds (by sap indexing) and 29% of blueberry seeds (by ELISA) (A. M. Childress-Roberts & D. C. Ramsdell, unpublished data). It is not known if the virus is transmitted to the seed through pollen.
Serology
The virus is an excellent immunogen. A rabbit given intramuscular injections of 0.5 to 1.0 mg virus (MI strain) three or four times at 7-14 day intervals yielded antiserum samples with titres of 1/512-1/1024 in agar double diffusion tests (Ramsdell & Stace-Smith, 1979). The virus, in crude sap from C. quinoa , forms a single precipitin band in agar gel-diffusion tests. It is readily detected in blueberry tissue by ELISA (A. M. Childress-Roberts & D. C. Ramsdell, unpublished data).
Relationships
The biological and physicochemical properties of blueberry leaf mottle virus place it in the nepovirus group (Ramsdell & Stace-Smith, 1981). In agar gel double diffusion tests, purified preparations of the MI strain did not react with antisera to the following nepoviruses: arabis mosaic, artichoke Italian latent, cherry leaf roll (cherry and elderberry strains), cherry rasp leaf, grapevine fanleaf, peach rosette mosaic, raspberry ringspot, strawberry latent ringspot, tobacco ringspot, tomato black ring and tomato ringspot viruses (Ramsdell & Stace-Smith, 1979). However, purified preparations of the MI and NY strains reacted weakly with antiserum to grapevine Bulgarian latent virus, the heterologous titres for both strains being 1/128, whereas the homologous titre was 1/2048; antiserum to the MI strain had a homologous titre of 1/1024, and heterologous titres of 1/512 against the NY strain and 1/128 against grapevine Bulgarian latent virus. In tests with sap from infected C. quinoa, the MI and NY strains showed reactions of identity against antiserum to the MI strain, but against antiserum to the NY strain the NY strain formed a slight spur with the MI strain (Ramsdell & Stace-Smith, 1979). These results indicate that the MI and NY strains are almost identical and that the NY strain is too distantly related to grapevine Bulgarian latent virus to be considered merely a strain of that virus, as suggested by Uyemoto et al. (1977) and in Description No. 186.
Stability in Sap
The MI strain was infective in sap of C. quinoa held at room temperature (c. 20°C) for 5 but not 7 days, at 4°C for 14 but not 21 days, or after heating for 10 min at 65°C but not 70°C. Infectivity in C. quinoa sap survived dilution to 10-4, but not 10-5 (Ramsdell & Stace-Smith, 1979).
Purification
The MI and NY strains required different purification methods for maximum yield (Ramsdell & Stace-Smith, 1981). All steps should be performed at 0-4°C.
MI strain. Harvest well-infected C. quinoa leaves 7-10 days after inoculation. Homogenize in 2 ml/g cold 0.05 M boric acid-borax buffer (pH 7), containing 0.1 % (w/v) each of sodium thioglycollate (TGA) and sodium diethyldithiocarbamate (DIECA). Squeeze extract through cheesecloth, freeze overnight, and thaw at 4°C. Centrifuge thawed extract at low speed for 15 min, retaining supernatant fluid. Add chloroform and n-butanol, each to 10% (v/v), and stir for 15 min. After low-speed centrifugation, collect the aqueous supernatant fluid. Add polyethylene glycol (PEG, M. Wt 6000) to 8% (w/v) and NaCl to 1% (w/v). After the PEG dissolves, centrifuge the mixture at low speed for 15 min and resuspend the pellet in 10% of the starting volume with 0.05 M Tris-HCl buffer, pH 7.4. Allow suspension to stand several hours. Centrifuge at low speed for 15 min and retain the supernatant fluid. Centrifuge at 137,000 g for 90 min. Resuspend pellet overnight in 0.05 M Tris-HCl buffer, pH 7.4. Further purify by centrifugation in a 5-30% sucrose gradient in a Beckman SW 41 rotor for 90 min at 38,000 rev./min. Collect the combined middle (M) and bottom (B) components (usually poorly separated), dilute three or four-fold with buffer and centrifuge at 137,000 g for 3 h. Resuspend pellet in 0.05 M Tris-HCl buffer, pH 7.4. Average yield is c. 0.5 mg/100 g infected C. quinoa leaves.
NY strain. Extract C. quinoa leaves in 2 ml/g 0.5 M boric acid-borax (pH 7), containing 0.1% (w/v) each of TGA and DIECA. Freeze extract overnight and after thawing at 4°C, centrifuge at low speed. Add ammonium sulphate to the supernatant fluid to 20% (w/v) and stir for 6 h. Centrifuge at low speed for 15 min. Discard the sediment and centrifuge the supernatant fluid at 108,000 g for 2.5 h. (Note: ammonium sulphate apparently interferes with precipitation of the virus by PEG; therefore, ultracentrifugation is necessary at this stage). Resuspend pellet overnight in 0.05 M Tris-HCl buffer, pH 7.4. Centrifuge at low speed for 15 min. Further purify by passing through sucrose gradients as above.
Properties of Particles
The virus particles sediment as three components (T, M and B) in sucrose density gradients (Ramsdell & Stace-Smith, 1979, 1981).
Sedimentation coefficient, s20,w (svedbergs): 53 (T), 120 (M), 128 (B). The MI strain has consistently more M component than B component, whereas the converse is true for grapevine Bulgarian latent virus (Fig. 6). Particles of the NY strain consistently fail to resolve into M and B components in sucrose gradients (Fig. 6).
Isoelectric point: pH 7.5.
Electrophoretic mobility: 0.168 x 10-5 cm2 sec-1 volt-1 at pH 7.0 in 0.02 M Na2HPO4, 0.02 M Tris-citrate buffer. Determinations were made in 0.7% agarose slab gels. The virus migrates as a single electrophoretic component (D. C. Ramsdell & J. M. Gillert, unpublished data).
Absorbance at 260 nm (1 mg/ml, 1 cm light path): 10.0 (M & B components combined).
A260/A280 (unfractioned virus) = 1.69 (not corrected for light-scattering).
Buoyant density in CsCl (g/cm3): (M) 1.471; (B) 1.497.
Particle Structure
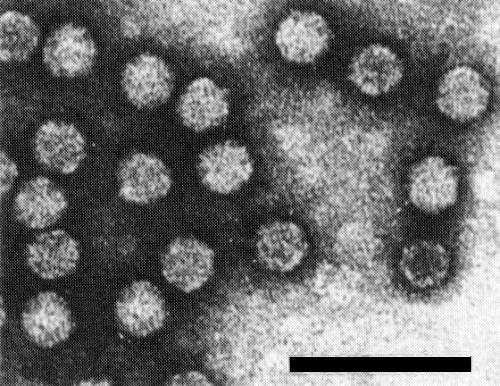
Isometric particles c. 28 nm in diameter (Fig. 8) (Ramsdell & Stace-Smith, 1979). T particles are penetrated by negative stain.
Particle Composition
(Ramsdell & Stace-Smith, 1981).
Nucleic acid: RNA, single-stranded. Two species, of M. Wt 2.15 x 106 and 2.35 x 106 (determined under non-denaturing conditions in 2.4% polyacrylamide gels) are found in M and B components, respectively. Unfractionated RNA preparations have a Tm of 60°C and show 15.4% hyperchromicity when heated from 30 to 99°C in 1 x SSC buffer, pH 7.0. M and B components contain 37 and 40-41% RNA, respectively, based on calculations from buoyant density in CsCl (Sehgal et al. , 1970).
Protein: Subunits have M. Wt of c. 54,000, estimated by electrophoresis in 5.0% SDS-polyacrylamide gels.
Relations with Cells and Tissues
(D. C. Ramsdell & R. Stace-Smith, unpublished data). In diseased blueberry the virus is present in leaves and blossoms throughout the bush. Roots have not been tested. The virus was readily detectable by ELISA in pollen from infected bushes (A. M. Childress-Roberts & D. C. Ramsdell, unpublished data). In infected C. quinoa leaves (Fig. 7), virus-like particles are found in aggregates in the cytoplasm of parenchyma cells, in close proximity to vesicles of the endoplasmic reticulum but not in association with other cell organelles.
Notes
The vector of blueberry leaf mottle virus remains unknown. It is suspected that the virus spreads in blueberry fields via pollen. The pattern of spread is not patchy like that of a typical nepovirus, but rather more random. In preliminary tests in which virus-free 2-year-old blueberry plants were caged with established infected plants in the field together with honeybees, a few berries (seeds) on the healthy test plants contained blueberry leaf mottle virus at harvest, as assayed by ELISA, but the pedicels did not (A. M. Childress-Roberts and D. C. Ramsdell, unpublished data).
Figures

Necrotic ringspots in inoculated leaves of Nicotiana clevelandii and pin-point necrotic lesions in uninoculated terminal leaves 4 days post-inoculation.

Linear-log sucrose density gradient profiles of a) strain MI, b) grapevine Bulgarian latent virus, c) strain NY. Equal amounts of purified virus were applied to gradients and centrifuged in a Beckman SW41 rotor at 4°C, 38,000 rev./min for 90 min.

Cross-section through parenchyma cell of a C. quinoa leaf infected with isolate MI. Virus-like particles (VLP) occur in the cytoplasm in aggregates amongst endoplasmic reticulum vesicles (ERV), and are not associated with other sub.cellular components. Bar represents 500 nm. (Courtesy of B. Schroeder, Agriculture Canada, Vancouver, British Columbia, Canada.)
References list for DPV: Blueberry leaf mottle virus (267)
- Ramsdell & Stace-Smith, Acta Horticulturae 95: 37, 1979.
- Ramsdell & Stace-Smith, Proc. 7th Meeting Int. Counc. for Study of Viruses and Virus-like Dis. of Grapevine, Niagara Falls, Canada, 1980: 119, 1980.
- Ramsdell & Stace-Smith, Phytopathology 71: 468, 1981.
- Sehgal, Jean, Bhalla, Soong & Krause, Phytopathology 60: 1778, 1970.
- Uyemoto, Taschenberg & Hummer, Pl. Dis. Reptr 61: 949, 1977.