Details of DPV and References
DPV NO: 292 July 1984
Family: Potyviridae
Genus: Potyvirus
Species: Papaya ringspot virus | Acronym: PRSV
This is a revised version of DPV 84
Papaya ringspot virus
D. Purcifull Departments of Plant Pathology and Agronomy, University of Florida, Gainesville, Florida 32611, USA
J. Edwardson Departments of Plant Pathology and Agronomy, University of Florida, Gainesville, Florida 32611, USA
E. Hiebert Departments of Plant Pathology and Agronomy, University of Florida, Gainesville, Florida 32611, USA
D. Gonsalves Department of Plant Pathology, Cornell University, New York State Agricultural Experiment Station, Geneva, New York 14456, USA
Contents
- Introduction
- Main Diseases
- Geographical Distribution
- Host Range and Symptomatology
- Strains
- Transmission by Vectors
- Transmission through Seed
- Transmission by Grafting
- Transmission by Dodder
- Serology
- Nucleic Acid Hybridization
- Relationships
- Stability in Sap
- Purification
- Properties of Particles
- Particle Structure
- Particle Composition
- Properties of Infective Nucleic Acid
- Molecular Structure
- Genome Properties
- Satellite
- Relations with Cells and Tissues
- Ecology and Control
- Notes
- Acknowledgements
- Figures
- References
Introduction
-
Described by Jensen (1949a), Conover (1962, 1964a), De Bokx (1965) and Webb & Scott (1965). A strain of this virus, watermelon mosaic virus 1, was confounded in Description No. 63 with watermelon mosaic virus 2).
Selected synonyms
- Papaya (papaw) distortion ringspot virus (Rev. appl. Mycol.
41: 530)
- Papaya (papaw) mosaic virus (Rev. appl. Mycol. 39: 147; Rev. appl. Mycol. 43: 1719)
- Specific watermelon mosaic virus (Rev. appl. Mycol. 46: 818)
- Watermelon mosaic virus 1 (Rev. appl. Mycol. 45: 318)
- Papaya (papaw) mosaic virus (Rev. appl. Mycol. 39: 147; Rev. appl. Mycol. 43: 1719)
-
A virus with RNA-containing, flexuous filamentous particles c. 780 x 12 nm. It induces cylindrical (pinwheel) inclusions and amorphous inclusions in the cytoplasm of host cells. It is transmissible mechanically, and by numerous species of aphid in a non-persistent manner, and has a narrow host range. Most strains belong to one of two major types, which are serologically very closely related. Type P (papaya-infecting) isolates cause an important disease in papaya and also occur in cucurbits. Type W (= watermelon mosaic virus 1) isolates cause important diseases in watermelon and other cucurbits, but do not infect papaya. Widely distributed throughout the world.
Main Diseases
In papaya, type P isolates cause mottling and distortion of leaves, rings and spots on fruit, and streaks on stems and petioles. Plants are stunted and fruit set is reduced (Conover, 1964a; Jensen, 1949a). Cool weather favours the development of severe leaf distortion symptoms (Jensen, 1949a; Conover, 1964a; Lima & Gomes, 1975). In squash, watermelon and other cucurbits, type W isolates cause mottling and distortion of leaves and fruit (Halliwell, Johnson & Cotner, 1979). Cucurbitaceous weeds (e.g. Melothria pendula and Momordica charantia) can serve as natural sources of type W isolates (Adlerz, 1972a, 1972b).
Geographical Distribution
Type P isolates occur in most tropical and subtropical areas where papaya is grown (Purcifull, 1972), including the USA (Conover, 1964a; Jensen, 1949a; Wan & Conover, 1983), South America (Herold & Weibel, 1962; Lima & Gomes, 1975), the Caribbean countries (Adsuar, 1947; Story & Halliwell, 1969), India (Capoor & Varma, 1958), Taiwan (Wang et al., 1978), Africa (Lana, 1980) and Okinawa (Yonaha, 1976). Type W isolates have been reported in cucurbits in many areas, including the USA (Webb, Bohn & Scott, 1965), Mexico (Milne, Grogan & Kimble, 1969), Caribbean countries (Schmelzer, 1969; Quiot, Kaan & Beramis, 1971), Australia (Greber, 1978), Germany (Hein, 1977), France (Lecoq, Lot & Pitrat, 1982), Italy (Ragozzino & Stefanis, 1977), India (Ghosh & Mukhopadhyay, 1979), Middle eastern countries (Ebrahim-Nesbat, 1974; Russo et al., 1979; Makkouk & Lesemann, 1980) and South America (Silva & Costa, 1978). An antigenic variant distinguishable from type P and type W isolates has been discovered in Guadeloupe (L. Quiot-Douine, J. E. Quiot & G. Labonne, unpublished data).
Host Range and Symptomatology
Transmissible by inoculation with sap, but host range is narrow. Fifteen dicotyledonous species in the families Caricaceae, Chenopodiaceae and Cucurbitaceae have been infected experimentally by type P isolates, with papaya (Jensen, 1949a; Conover, 1964a) and cucurbits (Wang et al., 1978) reported as natural hosts. Type W isolates are reported to infect 38 species in 11 genera of Cucurbitaceae, and two species of Chenopodiaceae, with squash, watermelon, cucumber and cantaloupe among the economically important natural hosts. The Guadeloupe strain infects various cucurbits (L. Quiot-Douine, J. E. Quiot & G. Labonne, unpublished data).
-
Diagnostic species
- Carica papaya
(papaya). Symptoms induced in papaya by type P isolates are variable and depend on stage of infection, plant vigour, temperature, virus strain and plant size (Conover, 1964a, 1964b). Seedlings show prominent vein clearing and downward cupping of the young leaves in about 1-2 weeks. After several weeks, the leaves become mottled and distorted, the lobes being markedly reduced in size (Fig. 1 and Fig. 2). Plant- to-plant variation in the expression of distortion symptoms was noted (Conover, 1964a). Spots or streaks with a greasy or water-soaked appearance sometimes occur on the stems (Jensen, 1949a). Fruit symptoms on plants in the field consist of spots or rings (Fig. 4). Fruit distortion also sometimes occurs. Type W isolates do not infect papaya (Milne & Grogan, 1969; Purcifull & Hiebert, 1979; Yeh, Gonsalves & Provvidenti, 1984). - Cucurbita pepo (zucchini squash, pumpkin). Some type P isolates
induce prominent mosaic and leaf distortion, whereas others induce mild mottle
(Gonsalves & Ishii, 1980).
Type W isolates typically induce foliar symptoms
consisting of mosaic (Fig. 8), dark green blisters and distortion. The
Guadeloupe strain causes stripe mosaic and distortion.
- Cucumis metuliferus (horned cucumber). Systemic mottle or mosaic symptoms are induced by type P and type W isolates in Accession 2459. Plant introduction P1 292190 is resistant to both types (Provvidenti & Robinson, 1977; Provvidenti & Gonsalves, 1982).
- Luffa acutangula. Chlorotic mottling or spotting on systemically infected leaves is induced by numerous type W isolates (Webb, 1965; Purcifull & Hiebert, 1979; Russo et al., 1979). A Florida isolate of type P also infects this species (Milne et al., 1969). Many watermelon mosaic virus 2 isolates (Purcifull, Hiebert & Edwardson, 1984b) do not infect L. acutangula, and this species has been used as a means of obtaining type W isolates free from watermelon mosaic virus 2 (Webb, 1965; Greber, 1978; D. Purcifull, unpublished data).
- Nicotiana benthamiana. Not susceptible to isolates of type P or type W. However, watermelon mosaic virus 2 invades this host systemically (Christie & Crawford, 1978; Purcifull & Hiebert, 1979; Russo et al., 1979).
- Cucumis metuliferus (horned cucumber). Systemic mottle or mosaic symptoms are induced by type P and type W isolates in Accession 2459. Plant introduction P1 292190 is resistant to both types (Provvidenti & Robinson, 1977; Provvidenti & Gonsalves, 1982).
-
Propagation species
- Papaya, squash and C. metuliferus are suitable for maintaining cultures of type P isolates and the last-named species is useful as a source of virus for purification (Yeh et al., 1984). Type W isolates are maintained readily in squash or pumpkin, which are useful as hosts in which to propagate the virus for purification.
-
Assay species
- Type P isolates (Fig. 3) (Gonsalves & Ishii, 1980; Yeh et al., 1984) and many, but not all, isolates of type W (Russo et al., 1979; Yeh et al., 1984) induce necrotic local lesions in Chenopodium amaranticolor and C. quinoa. C. quinoa is useful as an assay host for detecting nitrous acid-induced mutants (Yeh & Gonsalves, 1984). Pumpkin and squash are useful hosts for vector studies.
Strains
Type P isolates infect papaya whereas type W isolates do not. Isolates of type P and type W are antigenically indistinguishable. A variant found in Guadeloupe is serologically related to, but distinct from, typical P and W isolates (L. Quiot-Douine, D. Purcifull & E. Hiebert, unpublished data). Isolates within type P differ in the severity of the symptoms they induce (Conover, 1964b; Gonsalves & Ishii, 1980). Mild isolates that cross-protect against severe ones in papaya have been detected following nitrous acid treatment of virus (Yeh & Gonsalves, 1984). Such avirulent forms have potential as a means of disease control by cross-protection. A strain that induces wilt in papaya has been noted in Taiwan (Chang, 1979). Some type W isolates induce lesions in C. quinoa or C. amaranticolor whereas others do not (Russo et al., 1979).
Transmission by Vectors
Transmitted in a non-persistent manner by numerous species of aphid. Type P
isolates are transmitted by 21 species in 11 genera, including Myzus persicae
(Jensen, 1949b;
Conover, 1964a;
Zettler, Edwardson & Purcifull, 1968)
and Aphis gossypii. Type W isolates are transmitted by
24 species in 15 genera, including Myzus persicae, Aulacorthum solani, Aphis
craccivora and Macrosiphum euphorbiae
(Karl & Schmelzer, 1971).
The virus was transmitted by M. persicae following acquisition probes of
15-45 s
(Milne & Grogan, 1969), and by M. persicae and Aphis
citricola following acquisition probes of 10-60 s and inoculation access
periods of 1 h
(Adlerz, 1974).
Leafminer flies (Liriomyza sativae) transmitted two type W isolates from squash to squash in greenhouse trials, but transmission frequencies were low (Zitter & Tsai, 1977).
Transmission through Seed
None detected in papaya (Ishii & Holtzmann, 1963; Conover, 1964a; Wang et al., 1978). No transmission of type W isolates was detected in Cucurbita (Camino Lavin, Téliz & Soso Moss, 1974; Hein, 1977).
Serology
The virus is a good immunogen. Liquid immunoprecipitin tests with clarified
plant extracts
(Webb & Scott, 1965)
or with purified virus particles
(Milne & Grogan, 1969)
have been used for virus detection and for studying
relationships. The intact virus particles do not diffuse readily in agar gels
but sodium dodecyl sulphate (SDS)
(Purcifull & Hiebert, 1979) or pyrrolidine
(Shepard, Secor & Purcifull, 1974)
can be added to disrupt the particles
into diffusible fragments. SDS-immunodiffusion tests have been used to detect
isolates of types P and W in cucurbit leaf extracts with antisera to untreated
virus (Fig. 9)
(Purcifull & Hiebert, 1979;
Russo et al., 1979) or with
antisera to SDS-treated particle protein
(Gonsalves & Ishii, 1980).
Freeze-drying of leaf extracts is a convenient method for preparing reference
antigens for use in SDS-immunodiffusion tests
(Purcifull & Hiebert, 1979).
Virus detection in papaya by SDS-immunodiffusion is possible (Fig. 9) but
unreliable
(Wan & Conover, 1983) unless 5% ascorbic acid is added to the leaf
extracts (S.-D. Yeh & D. Gonsalves, unpublished data). Enzyme-linked
immunosorbent assay (ELISA) has been used to detect the virus; much higher
A405 readings are obtained when extracts from infected papaya
or squash leaves are prepared in 0.25 M potassium phosphate + 0.1 M EDTA, pH 7.5,
than in extracts prepared in standard phosphate-buffered saline
(Gonsalves & Ishii, 1980).
Immunoelectron microscopic procedures have been used to study
relationships
(Makkouk & Lesemann, 1980;
Samah, 1982).
Antisera have been prepared to the proteins of cylindrical (pinwheel) inclusions (CI) and amorphous inclusions (AI). The CI proteins are detectable by SDS-immunodiffusion methods (Baum, 1980; Baum & Purcifull, 1981; Yeh, 1984). Antisera prepared to purified CI proteins of type P and type W isolates had titres of 1/8 to 1/16 in SDS-immunodiffusion tests. With indirect ELISA, CI proteins were detected at concentrations as low as 1 .6 ng/ml and at sap dilutions of 3.2 x 105 (Yeh, 1984). AI protein of a type W isolate was detectable by SDS-immunodiffusion tests, by Western blotting tests, and by immunofluorescence in situ (M. De Mejia, E. Hiebert & D. Purcifull, unpublished data).
Relationships
Its particle morphology, aphid transmissibility, serological relationships
and ability to induce pinwheel inclusions in host cells place papaya ringspot
virus in the potyvirus group
(Matthews, 1982;
Hollings & Brunt, 1981).
Edwardson (1974) assigned both type P
and type W isolates to subgroup I because
they typically induce pinwheels and scrolls, but not laminated aggregates, in host
cells.
Type W isolates of papaya ringspot virus were called watermelon mosaic virus 1 by
Webb & Scott (1965);
however, the coat proteins of these isolates are
serologically very closely related, if not identical, to those of type P isolates
(Fig. 9)
(Purcifull & Hiebert, 1979;
Russo et al., 1979;
Gonsalves & Ishii, 1980;
Barbosa & Paguio, 1982;
Yeh et al., 1984) as also are
their CI proteins
(Baum, 1980;
Yeh, 1984). Also, in cross-protection tests, mild
type P isolates protect not only against severe type P isolates but also against
type W isolates
(Yeh & Gonsalves, 1984). Moreover, isolates of type P and type
W are controlled by the same resistance gene in Cucumis metuliferus, and
genotypes of C. melo, C. sativus, Lagenaria siceraria and Cucurbita
ecuadorensis that are resistant to isolates of type W are also resistant to
those of type P
(Provvidenti & Gonsalves, 1982). The properties of type P and
type W isolates, and their biological and cytological similarities, justify their
grouping here under one name, papaya ringspot virus, as suggested by
Lovisolo (1980).
Webb & Scott (1965)
also identified a second type of isolate (which they
called watermelon mosaic virus 2) distinguishable from watermelon mosaic virus 1
by serological, cross-protection and host range tests. A contradictory report by
Milne & Grogan (1969)
led to these two viruses being included together in
Description No. 63. However, there is now much evidence that watermelon mosaic
virus 2 is serologically very different, not only from type W (= watermelon
mosaic virus 1), but also from type P isolates of papaya ringspot virus
(Fig. 9)
(Greber, 1978;
Purcifull & Hiebert, 1979;
Russo et al., 1979;
Gonsalves & Ishii, 1980;
Yeh et al., 1984),
although distant relationships have been detected
(Purcifull & Hiebert, 1979, and unpublished
data;
Makkouk & Lesemann, 1980;
Samah, 1982;
Dodds et al., 1984).
Also, the CI induced by watermelon mosaic virus 2 are antigenically distinct
from those induced by type P and type W isolates of papaya ringspot virus
(Baum & Purcifull, 1981;
Yeh, 1984).
Moreover, no nucleotide sequence homology was
detected by molecular hybridisation between cDNA prepared from a type W isolate
of papaya ringspot virus and RNA molecules of watermelon mosaic virus 2
(Samah, 1982);
nor was any such homology detected between the RNA molecules of
lettuce mosaic,
potato Y,
bean yellow mosaic,
soybean mosaic or
bean common mosaic viruses
(Samah, 1982).
Watermelon mosaic 2, is now the subject of a separate,
revised Description (No. 293).
Papaya ringspot virus has affinities with some other potyviruses. Pyrrolidine degradation products of its particles reacted with antiserum to the D-protein of tobacco etch virus (Shepard et al., 1974), and the particle proteins and CI proteins of a potyvirus isolated from cucurbits in Morocco (Fischer & Lockhart, 1974) are serologically related to, but distinct from, those of papaya ringspot virus (Baum, 1980; Baum & Purcifull, 1981). The relationship of papaya ringspot virus to certain watermelon mosaic virus isolates from the USA (Anderson, 1954) and South Africa (Van Regenmortel, 1971) is unclear.
Stability in Sap
Infectivity of the virus in papaya sap was lost after 8 h at room temperature, after heating to 54-56°C for 10 min and by dilution to 10-3 (Conover, 1964a). In squash sap, type W isolates were inactivated by aging for 40-60 days, by heating to 60°C for 10 min or by dilution to 5 x 10-4 (Webb & Scott, 1965). Type W isolates retained infectivity after storage for at least 6 years in dried leaf pieces over CaCl2 under vacuum at 4°C (D. Purcifull, unpublished data).
Purification
Purification of virus particles (method 1).
(Gonsalves & Ishii, 1980).
Inoculate Cucumis metuliferus accession 2459
(Yeh et al., 1984)
or zucchini squash at cotyledonary stage and harvest systemically
infected leaves after 21-25 days. Homogenise in a blender with 0.5 M potassium
phosphate, pH 7.5 (2 ml/g tissue), containing 0.01 M ethylenediamine-
tetraacetate (EDTA) and 0.1% sodium sulphite. Add 0.5 ml each of chloroform
and carbon tetrachloride per g tissue during homogenisation. Centrifuge
homogenate at 10,000 g for 10 min. Recover the aqueous phase and,
to each 100 ml, add 8 g of polyethylene glycol (PEG) M. Wt 6000, stir 1-4 h at
6°C, centrifuge at 10,000 g for 10 min and resuspend in 20
ml 0.1 M potassium phosphate containing 0.01 M EDTA, pH 7.0 (PE buffer).
Centrifuge at 10,000 g for 10 min, and re-precipitate the virus
from the supernatant fluid by adding PEG and NaCl to final concentrations of
5% and 0.3 M, respectively. Centrifuge at 10,000 g for 10 min,
and resuspend the pellet in 5 ml PE buffer. Further purify by dissolving 0.15
g Cs2SO4 in each ml of virus suspension, layer on a cushion
of 53% (w/w) Cs2SO4 in PE buffer, centrifuge 18-22 h at
30,000 rev/min at 6°C. Collect virus-containing zone, dilute with 2 vol. PE
buffer, remove host material by centrifugation at 10,000 g for 10
min, and recover the supernatant fluid, which contains the virus. Dialyse
against PE buffer overnight to remove caesium salts. Further purification can
be achieved by recentrifugation in Cs2SO4. Yields are
about 5 mg per 100 g tissue.
Purification of virus particles (method 2). (Modification of
method used by
Purcifull & Hiebert (1979) for a type W isolate). Homogenise
Small Sugar pumpkin tissue (100 g), collected 3-4 weeks after inoculation, in
cold 200 ml 0.5 M potassium phosphate buffer (pH 7.5) (PB) containing 0.1% sodium
sulphite, 50 ml chloroform and 50 ml carbon tetrachloride. Centrifuge at 1000
g for 5 min. Re-extract the pellet with 100 ml PB and centrifuge
again. Combine the supernatant fluids and centrifuge at 13,000 g
for 15 min. The resulting pellet can be used for purifying cylindrical inclusions
(Hiebert, Purcifull & Christie, 1984a).
To the supernatant fluid (which contains the virus), add Triton X-100 to 1% (v/v), PEG (M. Wt 6000-8000)
to 4% (w/v) and NaCl to 100 mM, and stir for 1 h at 4°C. Centrifuge at
10,000 g for 10 min. Resuspend the pellets in 50 ml 20 mM PB with
the aid of a tissue grinder. Centrifuge at 12,000 g for 10 min and
discard the pellet. Re-precipitate virus particles from the supernatant fluid by
adding PEG to 8% (w/v) and NaCl to 100 mM and stir for 0.5 h. Centrifuge at
12,000 g for 10 min. Resuspend the pellets in 5-10 ml 20 mM PB
with the aid of a tissue grinder. Layer the resuspended material over 30% (w/w)
CsCl or Cs2SO4 in 20 mM PB and centrifuge at 140,000
g for 16-18 h at 5°C. Collect the virus-containing zone by
droplet fractionation, dilute with an equal volume of 20 mM PB and then
centrifuge at 12,000 g for 10 min. Recover the virus from the
supernatant fluid by PEG precipitation as before. The yield of virus particles
is about 5-10 mg/100 g tissue. Clarification with n-butanol and the use of 10
mM EDTA in the buffer during purification (both useful for watermelon mosaic
virus 2 purification) are detrimental to type W isolates (E. Hiebert,
unpublished data).
Purification of cylindrical (pinwheel) inclusion (CI) protein.
(Yeh, 1984).
Propagate the virus in zucchini squash. Monitor the development and abundance of
CI by using light microscopy of epidermal strips stained with luxol brilliant
green-calcomine orange
(Christie & Edwardson, 1977). Collect the tissue
approximately 25-30 days after inoculation. Homogenise each 100 g tissue in
a blender for 2 min in 200 ml 0.5 M potassium phosphate buffer, pH 7.5,
containing 0.25% sodium sulphite. Add 50 ml chloroform and 50 ml carbon
tetrachloride and homogenise for 1 min. Centrifuge at 165 g
for 5 min save the upper aqueous phase and re-extract the pellets with 100
ml buffer. Centrifuge at 165 g for 5 min and combine the
supernatant fluid with the aqueous phase already retained. Centrifuge at 1000
g for 5 min, discard the pellets, and centrifuge the supernatant
fluid at 10,000 g for 20 min. The supernatant fluid after this
step can be used for virus purification. To purify the CI, resuspend the
pellets in 50 ml 0.05 M potassium phosphate buffer, pH 8.2, containing 0.1%
2-mercaptoethanol (2-ME), with the aid of a homogeniser. Add Triton X-100 to
a final concentration of 5%, stir 1 h at 4°C, and centrifuge at 38,000
g for 15 min. Resuspend the pellets in 5 ml 0.02 M potassium
phosphate buffer, pH 8.2, containing 0.1% 2-ME using a homogeniser. Repeat
treatment with Triton X-100 and high speed centrifugation, and resuspend
pellet in 2 ml 0.02 M Tris-HCl, pH 8.2.
CI proteins can be assayed by SDS-polyacrylamide gel electrophoresis. Further
purify the CI protein by preparatory slab gel electrophoresis
(Laemmli, 1970),
degrading the CI by boiling for 2 min in 0.33 vol. buffer (0.4 M Tris-HCl, pH
6.8, 10% SDS, 20% 2-ME, 20% sucrose and 0.005% bromophenol blue). Centrifuge for
5 min at low speed to remove undenatured material, conduct electrophoresis, and
then make the band visible by soaking the gel in 0.2 M KCl for c. 10 min
at 4°C. Excise the band, rinse three times for 5 min in cold distilled
water and store frozen. To elute protein, thaw gels, cut in 1 mm slices, place
in 10 vol. buffer (0.05 M Tris-HCl, pH 7.9, 0.1% SDS, 0.1 mM EDTA and 0.15 M
NaCl), and stir at room temperature for 6-8 h. Remove liquid and save; repeat
process. Pool the two eluates, filter through paper to remove gel pieces, place
sample on ice, and precipitate the CI protein by adding trichloracetic acid to
a final concentration of 20% (w/v). After 10-15 min centrifuge at 12,000
g for 10 min, rinse the pellet with anhydrous ethyl ether,
resuspend in 6.0 M guanidine-HCl in 0.02 M Tris-HCl, pH 8.2. Store the CI
protein frozen or at 6°C with sodium azide.
Purification of amorphous inclusion body (AI) protein. (De Mejia, 1984). Inoculate zucchini squash in cotyledonary stage and harvest systemically infected tissue 4-6 weeks later. Homogenise the tissue in a blender in 1 ml extraction buffer (EB) (0.1 M Tris, pH 7.5, 0.5% sodium sulphite) per g tissue. Filter through 2 layers of cheesecloth, layer over 30 ml 20% sucrose in EB, and centrifuge at 4000 g for 5 min (Sorvall GSA rotor). Resuspend the pellets in cold EB, add Triton X-100 to a final concentration of 5%, and stir 1 h at 4°C. Centrifuge at 4000 g for 5 min through 15 ml 40% sucrose in EB, and resuspend the pellets in EB. Repeat the Triton X-100 treatment and centrifugation in 40% sucrose two more times, and resuspend in a small volume of 0.1 M Tris, pH 7.5. Monitor presence of AI in extracts by light microscopy of preparations stained with 0.15% phloxine in water. Store inclusions frozen. Purify inclusion protein by preparatory gel electrophoresis (Laemmli, 1970), dissociating the inclusions by boiling in 0.1 M Tris-HCl, pH 6.8, containing 2.5% SDS, 5% 2-ME and 5% sucrose. Detect AI protein in the gel by pre-labelling a portion of the AI protein with dansyl chloride (Talbot & Yphantis, 1971). Excise the gel band, crush with mortar and pestle, add 10 vol. water, freeze at -20°C for several hours, incubate at room temperature for several hours, and centrifuge at 1000 g for 5 min. Re-extract the pellet, re-centrifuge, combine the two supernatant fluids, filter through 0.45 µm Millipore membrane, and freeze-dry to remove water. Resuspend dried residue in 2 ml deionized water and dialyse for 8 h against deionized water. Store at -20°C. Yields up to 35 A280 units per kg tissue are obtained.
Properties of Particles
A260/A280: 1.2 (uncorrected for
light-scattering; E. Hiebert, unpublished data).
Buoyant density in CsCl: 1.32 g/cm3 (De La Rosa & Lastra, 1983).
Particle Structure
Particles are flexuous filaments (Fig. 10) c. 12 nm in diameter and 760-800 nm long (Herold & Weibel, 1962; Purcifull & Edwardson, 1967; Purcifull & Hiebert, 1979; Russo et al., 1979; De La Rosa & Lastra, 1983).
Particle Composition
Nucleic acid: RNA, single-stranded (De La Rosa & Lastra, 1983). RNA extracted from virus particles by the procedure of Brakke & Van Pelt (1970) sediments at 39 S, like that of other potyviruses (E. Hiebert, unpublished data).
Protein: Subunit M. Wt 3.6-3.65 x 104. Two smaller products, which have M. Wt of 3.1-3.4 x 104 and 2.6-2.7 x 104 have been reported, and preparations stored 2 months contained primarily the smaller form. Presumably, the smaller form arises by proteolytic degradation (Purcifull & Hiebert, 1979; Gonsalves & Ishii, 1980; Baum, 1980).
Genome Properties
The RNA molecules of papaya ringspot virus types P and W are translated efficiently in vitro, and antisera to virus-specified structural and inclusion body proteins have been used to analyse the translation products (De Mejia, 1984; Hiebert, Thornbury & Pirone, 1984b; De Mejia, Hiebert & Purcifull, 1984; M. De Mejia, E. Hiebert & D. Purcifull, unpublished data; L. Quiot-Douine, D. Purcifull & E. Hiebert, unpublished data). The translation product profile is similar to those described for tobacco etch and pepper mottle viruses (Dougherty & Hiebert, 1980) except that products reactive with antiserum to the 49 x 103 M. Wt nuclear inclusion protein of tobacco etch virus are not detected. The proposed genetic map of the papaya ringspot virus genome (Fig. 11) is based on the in vitro translation analysis and its similarity to the tobacco etch virus analysis. In the rabbit reticulocyte lysate system, the predominant in vitro translation product, mapped near the 5' end, has an estimated M. Wt of 110 x 103 (for a type W isolate), 112 x 103 (for a type P isolate), or 114 x 103 (for the Guadeloupe isolate). This 110-114 K product is thought, by comparison with those formed in the wheatgerm system, to be a ‘read-through’ product consisting of a 40-60 K protein (not yet identified in vivo) and a 51 K protein, which reacts with antiserum to the AI protein of a type W isolate and to the helper component protein of tobacco vein mottling virus (M. de Mejia, E. Hiebert & D. Purcifull, unpublished data). Peptide mapping showed that the predominant (M. Wt 110 x 103) product of the type W isolate was distinct from that (M. Wt 107 x 103) of a watermelon mosaic virus 2 isolate (E. Hiebert, unpublished data) even though both products are immunoprecipitated by antiserum to the helper component of tobacco vein mottling virus (Hiebert et al., 1984b).
Relations with Cells and Tissues
Isolates of types P and W both typically induce two types of cytoplasmic
inclusion in their hosts: cylindrical inclusions (CI)
(Fig. 5 and Fig. 7) and amorphous
inclusions (AI)
(Fig. 6 and Fig. 7). The CI are striated, with a periodicity of 5 nm, and
are assocated with scrolls
(Purcifull & Edwardson, 1967;
Edwardson, Purcifull & Christie, 1968;
Zettler et al., 1968;
Martelli & Russo, 1976).
The CI aggregate to form fibrous structures that are clearly visible by light
microscopy of epidermal strips stained with luxol brilliant green-calcomine
orange
(Fig. 7). They contain a protein of M. Wt 6.9-7.0 x 104
(Baum, 1980;
Yeh, 1984)
which is distinct serologically from host protein and virus
particle protein.
The AI have been observed in tissues of papaya and cucurbits infected with various isolates (Edwardson, 1974; Martelli & Russo, 1976; Christie & Edwardson, 1977; Russo et al., 1979). However, the AI were not detected in tissues infected with one type W isolate, even though the AI protein was detected serologically (M. De Mejia, R. Christie, D. Purcifull & E. Hiebert, unpublished data). In some reports, the AI have been referred to as irregular cytoplasmic inclusions (Christie & Edwardson, 1977). The AI often are visible in the same cells as the CI (Martelli & Russo, 1976). The staining reactions of the inclusions indicate they contain both RNA and protein (Martelli & Russo, 1976; Christie & Edwardson, 1977). A major protein isolated from the AI has a M. Wt of 5.1 x 104; an antiserum to this protein reacts with AI in situ in immunofluorescence tests, and with the predominant in vitro translation product (mapped near the 5' end) of viral RNA (M. De Mejia, E. Hiebert & D. Purcifull, unpublished data; De Mejia et al., 1984).
Notes
The mosaic disease of papaya caused by papaya ringspot virus
(Ishii & Holtzmann, 1963)
should not be confused with that caused by papaya mosaic virus,
which is a potexvirus
(Purcifull & Hiebert, 1971). The two viruses can be
distinguished readily by particle morphology
(De Bokx, 1965), by the types of
intracellular inclusion they induce in their hosts
(Zettler et al., 1968;
Christie & Edwardson, 1977), and by serological tests
(Fig. 9)
(De Bokx, 1965;
D. Purcifull, unpublished data).
An aphid-transmitted, filamentous virus (c. 760 nm long) is associated
with yellow mottling and necrosis of papaya in Okinawa
(Yonaha, 1977); its
relationship to papaya ringspot virus is not clear. Other viruses that infect
papaya naturally
(Cook, 1972) include tobacco ringspot
(Lambe, 1963;
Lana, 1980), tomato spotted wilt
(Trujillo & Gonsalves, 1967), and one or more
rhabdoviruses
(Lastra & Quintero, 1981;
Wan & Conover, 1981), all of
which can be distinguished from papaya ringspot virus on the basis of particle
morphology.
Papaya ringspot virus isolates can be distinguished from
watermelon mosaic virus 2
(Webb & Scott, 1965;
Purcifull et al., 1984b) by
inclusion body morphology
(Edwardson, 1974;
Christie & Edwardson, 1977;
Russo et al., 1979) and by serology and host range
(Webb & Scott, 1965;
Purcifull & Hiebert, 1979;
Russo et al., 1979).
The following four
potyviruses
that also cause diseases of cucurbits can be
distinguished from papaya ringspot virus on the basis of SDS-immunodiffusion
tests: bean yellow mosaic virus
(Provvidenti & Uyemoto, 1973;
Purcifull & Hiebert, 1979);
a potyvirus from cucurbits in Morocco
(Fischer & Lockhart, 1974;
Purcifull & Hiebert, 1979;
Baum, Purcifull & Hiebert, 1979); zucchini yellow fleck virus
(Vovlas, Hiebert & Russo, 1981); and
zucchini yellow mosaic virus
(Lisa et al., 1981). The last-named virus
has been observed in cucurbits in various parts of the world
(Lecoq, Lisa & Dellavalle, 1983;
Lisa & Lecoq, 1984), sometimes in mixed infections
with other potyviruses, including type W isolates of papaya ringspot virus
(Purcifull et al., 1984a).
Numerous other viruses cause diseases of cucurbits (Lovisolo, 1980). Cucumber mosaic virus (Francki, Mossop & Hatta, 1979) and squash mosaic virus (Campbell, 1971) can be distinguished from papaya ringspot virus by host reactions (Grogan, Hall & Kimble, 1959; Milne et al., 1969), by SDS-immunodiffusion tests (Purcifull, Christie & Lima, 1981; Purcifull et al., 1984a) and by light microscopic examination of the types of inclusion they induce in host cells (Christie & Edwardson, 1977).
Figures

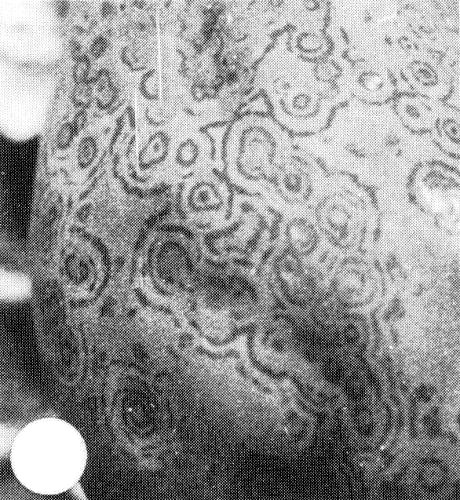
Distortion induced in papaya leaf by a type P isolate, showing lobes reduced to veins and small portions of laminar tissue.
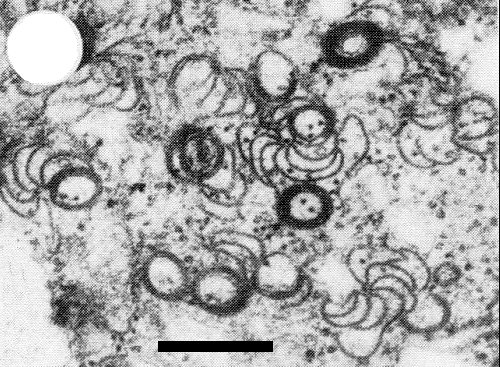
Electron micrograph of cylindrical (pinwheel) and scroll inclusions in cytoplasm of pumpkin leaf cell infected by a type W isolate. Bar represents 250 nm.

Electron micrograph of amorphous (irregular) inclusion body in cytoplasm of pumpkin leaf cell infected by a type W isolate. Bar represents 1 µm.

Light micrograph of pumpkin epidermal cell infected by a type W isolate, showing amorphous inclusion (ai) and a group of cylindrical (pinwheel) inclusions (ci) in the cytoplasm. Bar represents 10 µm. (Photomicrograph courtesy of R. G. Christie.)

Sodium dodecyl sulphate (SDS)-immunodiffusion tests illustrating close relationship of types P and W isolates of papaya ringspot virus and their distinction from certain other viruses. A = antiserum to a type W isolate. The other wells contain SDS-treated leaf sap: 1 = type W isolate in squash; 2= type P isolate in squash; 3 = healthy squash; 4 = watermelon mosaic virus 2 in squash; 5 = zucchini yellow mosaic virus in squash; 6 = type P isolate in papaya; 7 = healthy papaya; 8 = papaya mosaic virus (potexvirus) in papaya.

Electron micrograph of filamentous particles of a type P isolate, mounted in uranyl acetate (micrograph courtesy of S. R. Christie). Bar represents 200 nm.

Proposed genetic map of papaya ringspot virus (type P, Florida isolate). The M. Wt of the gene products are presented above the map and three of the products are identified below the map. The 112k product is serologically related to tobacco vein mottling virus helper component and the 57k product is serologically related to the 54k nuclear inclusion protein of tobacco etch virus.
References list for DPV: Papaya ringspot virus (292)
- Adlerz, J. econ. Ent. 65: 1303, 1972a.
- Adlerz, Pl. Dis. Reptr 56: 563, 1972b.
- Adlerz, Phytopathology 64: 350, 1974.
- Adsuar, J. Agric. Univ. P. Rico 31: 248, 1947.
- Anderson, Phytopathology 44: 198, 1954.
- Barbosa & Paguio, Fitopatol. Bras. 7: 37, 1982.
- Baum, Ph.D. Diss., Univ. Fla, 95 pp., 1980.
- Baum & Purcifull, Phytopathology 71: 202, 1981.
- Baum, Purcifull & Hiebert, Phytopathology 69: 1023, 1979.
- Brakke & van Pelt, Virology 42: 699, 1970.
- Camino Lavin, Téliz & Soso Moss, Agrociencia 18: 55, 1974.
- Campbell, CMI/AAB Descr. Pl. Viruses 43, 4 pp., 1971.
- Capoor & Varma, Indian J. agric. Sci. 28: 225, 1958.
- Chang, J. agric. Res. China 28: 207, 1979.
- Christie & Crawford, Pl. Dis. Reptr 62: 20, 1978.
- Christie & Edwardson, Monogr. Ser. Fla agric. Exp. Stn 9, 155 pp., 1977.
- Conover, Phytopathology 52: 6, 1962.
- Conover, Proc. Fla St. hort. Soc. 77: 440; 1964a.
- Conover, Proc. Fla St. hort. Soc. 77: 444, 1964b.
- Cook, Tech. Bull. Fla agric. Exp. Stn 750, 19 pp., 1972.
- De Bokx, Pl. Dis. Reptr 49: 742, 1965.
- De La Rosa & Lastra, Phytopath. Z. 106: 329, 1983.
- De Mejia, Ph.D. Diss., Univ. Fla, 52 pp., 1984.
- De Mejia, Hiebert & Purcifull, Phytopathology 74: 1015, 1984.
- Dodds, Lee, Nameth & Laemmlen, Phytopathology 74: 221, 1984.
- Dougherty & Hiebert, Virology 104: 183, 1980.
- Ebrahim-Nesbat, Phytopath. Z. 79: 352, 1974.
- Edwardson, Monogr. Ser. Fla agric. Exp. Stn 4, 398 pp., 1974.
- Edwardson, Purcifull & Christie, Virology 34: 250, 1968.
- Fischer & Lockhart, Pl. Dis. Reptr 58: 143, 1974.
- Francki, Mossop & Hatta, CMI/AAB Descr. Pl. Viruses 213, 6 pp., 1979.
- Ghosh & Mukhopadhyay, Phytopath. Z. 94: 172, 1979.
- Gonsalves & Ishii, Phytopathology 70: 1028, 1980.
- Greber, Aust. J. agric. Res. 29: 1235, 1978.
- Grogan, Hall & Kimble, Phytopathology 49: 366, 1959.
- Halliwell, Johnson & Cotner, Misc. Publn Tex. agric. Exp. Stn 1435, 5 pp., 1979.
- Hein, Phytopath. Z. 89: 221, 1977.
- Herold & Weibel, Virology 18: 302, 1962.
- Hiebert, Purcifull & Christie, in Methods in Virology Vol. 8, p. 225, eds K. Maramorosch & H. Koprowski, Academic Press: New York, 1984a.
- Hiebert, Thornbury & Pirone, Virology 135: 1, 1984b.
- Hollings & Brunt, CMI/AAB Descr. Pl. Viruses 245, 7 pp., 1981.
- Ishii & Holtzmann, Phytopathology 47: 947, 1963.
- Jensen, Phytopathology 39: 191, 1949a.
- Jensen, Phytopathology 39: 212, 1949b.
- Karl & Schmelzer, Arch. PflSchutz 7: 3, 1971.
- Laemmli, Nature, Lond. 227: 680, 1970.
- Lambe, J. Rio Grande Valley hort. Soc. 17: 128, 1963.
- Lana, J. hort. Sci. 55: 191, 1980.
- Lastra & Quintero, Pl. Dis. 65: 439, 1981.
- Lecoq, Lot & Pitrat, Agronomie 2: 787, 1982.
- Lecoq, Lisa & Dellavalle, Pl. Dis. 67: 824, 1983.
- Lima & Gomes, Fitossanidade, Fortaleza 1: 56, 1975.
- Lisa & Lecoq, CMI/AAB Descr. Pl. Viruses 282, 4 pp., 1984.
- Lisa, Boccardo, D'Agostino, Dellavalle & d'Aquilio, Phytopathology 71: 667, 1981.
- Lovisolo, Acta Hort. 88: 33, 1980.
- Makkouk & Lesemann, Pl. Dis. 64: 789, 1980.
- Martelli & Russo, Virology 72: 352, 1976.
- Matthews, Intervirology 17: 4, 1982.
- Milne & Grogan, Phytopathology 59: 809, 1969.
- Milne, Grogan & Kimble, Phytopathology 59: 819, 1969.
- Provvidenti & Gonsalves, J. Hered. 73: 239, 1982.
- Provvidenti & Robinson, J. Hered. 68: 56, 1977.
- Provvidenti & Uyemoto, Pl. Dis. Reptr 57: 280, 1973.
- Purcifull, CMI/AAB Descr. Pl. Viruses 84, 3 pp., 1972.
- Purcifull & Edwardson, Virology 32: 393, 1967.
- Purcifull & Hiebert, CMI/AAB Descr. Pl. Viruses 50, 4 pp., 1971.
- Purcifull & Hiebert, Phytopathology 69:112, 1979.
- Purcifull, Christie & Lima, Phytopathology 71: 1221, 1981.
- Purcifull, Adlerz, Simone, Hiebert & Christie, Pl. Dis. 68: 230, 1984a.
- Purcifull, Hiebert & Edwardson, CMI/AAB Descr. Pl. Viruses 293, 7 pp., 1984b.
- Quiot, Kaan & Beramis, Annls Phytopath. 3: 127, 1971.
- Ragozzino & Stefanis, Annali Fac. Sci. Agr. Univ. Napoli, IV, 11: 33, 1977.
- Russo, Martelli, Vovlas & Ragozzino, Phytopath. Medit. 18: 94, 1979.
- Samah, Ph.D. Diss., Univ. Adelaide, 109 pp., 1982.
- Schmelzer, in Plant Virology, p. 206, ed. C. Blattny, Academia: Prague, 346 pp., 1969.
- Shepard, Secor & Purcifull, Virology 58: 464, 1974.
- Silva & Costa, Summa Phytopath. 4: 71, 1978.
- Story & Halliwell, Pl. Dis. Reptr 53: 757, 1969.
- Talbot & Yphantis, Anal. Biochem. 44: 246, 1971.
- Trujillo & Gonsalves, Phytopathology 57: 9, 1967.
- Van Regenmortel, CMI/AAB Descr. Pl. Viruses 63, 4 pp., 1971.
- Vovlas, Hiebert & Russo, Phytopath. Medit. 20: 123, 1981.
- Wan & Conover, Proc. Fla St. hort. Soc. 94: 318, 1981.
- Wan & Conover, Pl. Dis. 67: 353, 1983.
- Wang, Wang, Chiu & Sun, Pl. Prot. Bull. 20: 133, 1978.
- Webb, Phytopathology 55: 1379, 1965.
- Webb & Scott, Phytopathology 55: 895, 1965.
- Webb, Bohn & Scott, Pl. Dis. Reptr 49: 532, 1965.
- Yeh, Ph.D. Diss., Cornell Univ., 98 pp., 1984.
- Yeh & Gonsalves, Phytopathology 74: 1086, 1984.
- Yeh, Gonsalves & Provvidenti, Phytopathology 74: 1081, 1984.
- Yonaha, Bull. CoIl. Agric. Univ. Ryukyus 23: 115, 1976.
- Yonaha, Bull. Coll. Agric. Univ. Ryukyus 24: 169, 1977.
- Zettler, Edwardson & Purcifull, Phytopathology 58: 332, 1968.
- Zitter & Tsai, Pl. Dis. Reptr 61: 1025, 1977.